Abstract
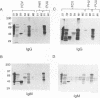
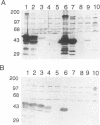
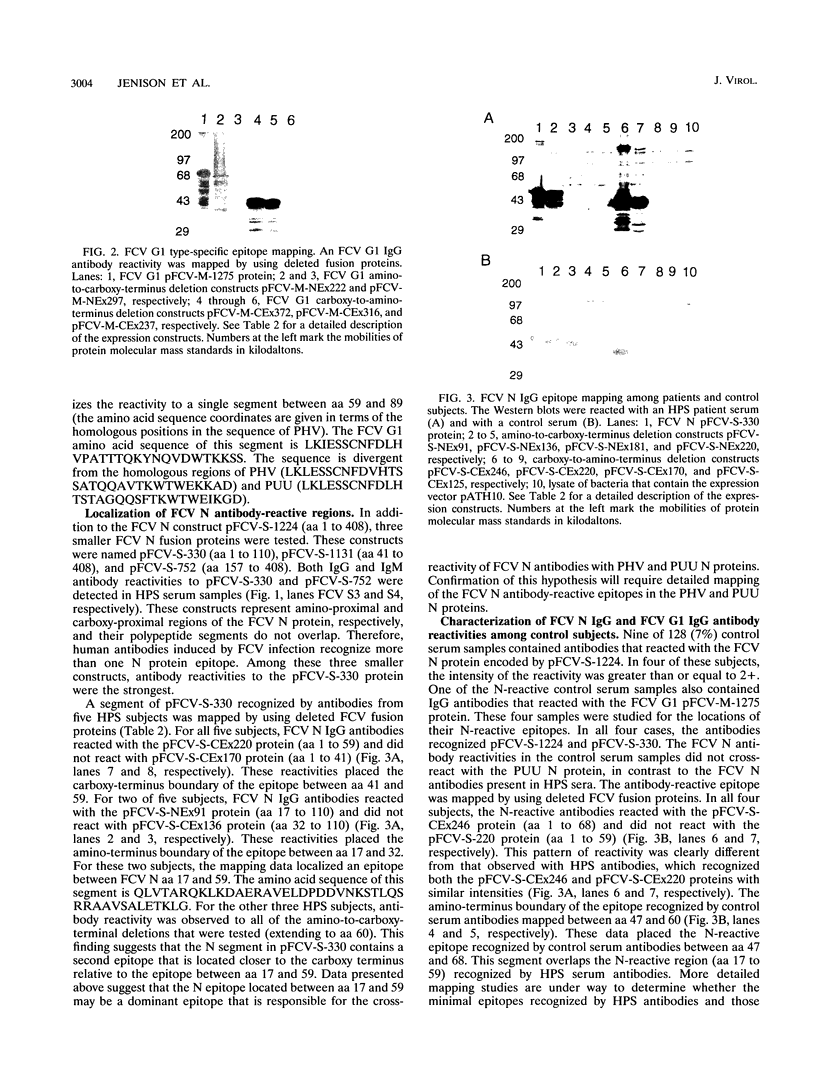
Hantavirus pulmonary syndrome (HPS) is a human disease caused by a newly identified hantavirus, which we will refer to as Four Corners virus (FCV). FCV is related most closely to Puumala virus (PUU) and to Prospect Hill virus (PHV). Twenty-five acute HPS serum samples were tested for immunoglobulin G (IgG) and IgM antibody reactivities to FCV-encoded recombinant proteins in Western blot (immunoblot) assays. All HPS serum samples contained both IgG and IgM antibodies to the FCV nucleocapsid (N) protein. FCV N antibodies cross-reacted with PUU N and PHV N proteins. A dominant FCV N epitope was mapped to the segment between amino acids 17 and 59 (QLVTARQKLKDAERAVELDPDDVNKSTLQSRRAAVSALETKLG). All HPS serum samples contained IgG antibodies to the FCV glycoprotein-1 (G1) protein, and 21 of 25 serum samples contained FCV G1 IgM antibodies. The FCV G1 antibodies did not cross-react with PUU G1 and PHV G1 proteins. The FCV G1 type-specific antibody reactivity mapped to a segment between amino acids 59 and 89 (LKIESSCNFDLHVPATTTQKYNQVDWTKKSS). One hundred twenty-eight control serum samples were tested for IgG reactivities to the FCV N and G1 proteins. Nine (7.0%) contained FCV N reactivities, 3 (2.3%) contained FCV G1 reactivities, and one (0.8%) contained both FCV N and FCV G1 reactivities. The epitopes recognized by antibodies present in control serum samples were different from the epitopes recognized by HPS antibodies, suggesting that the control antibody reactivities were unrelated to FCV infections. These reagents constitute a type-specific assay for FCV antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arikawa J., Schmaljohn A. L., Dalrymple J. M., Schmaljohn C. S. Characterization of Hantaan virus envelope glycoprotein antigenic determinants defined by monoclonal antibodies. J Gen Virol. 1989 Mar;70(Pt 3):615–624. doi: 10.1099/0022-1317-70-3-615. [DOI] [PubMed] [Google Scholar]
- Arikawa J., Yao J. S., Yoshimatsu K., Takashima I., Hashimoto N. Protective role of antigenic sites on the envelope protein of Hantaan virus defined by monoclonal antibodies. Arch Virol. 1992;126(1-4):271–281. doi: 10.1007/BF01309700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas J. R., Jr, Okuno Y., Asada H., Tamura M., Takahashi M., Tanishita O., Takahashi Y., Kurata T., Yamanishi K. Characterization of glycoproteins of viruses causing hemorrhagic fever with renal syndrome (HFRS) using monoclonal antibodies. Virology. 1986 Jun;151(2):379–384. doi: 10.1016/0042-6822(86)90058-9. [DOI] [PubMed] [Google Scholar]
- Groen J., Dalrymple J., Fisher-Hoch S., Jordans J. G., Clement J. P., Osterhaus A. D. Serum antibodies to structural proteins of Hantavirus arise at different times after infection. J Med Virol. 1992 Aug;37(4):283–287. doi: 10.1002/jmv.1890370409. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hjelle B., Jenison S., Torrez-Martinez N., Yamada T., Nolte K., Zumwalt R., MacInnes K., Myers G. A novel hantavirus associated with an outbreak of fatal respiratory disease in the southwestern United States: evolutionary relationships to known hantaviruses. J Virol. 1994 Feb;68(2):592–596. doi: 10.1128/jvi.68.2.592-596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenison S. A., Firzlaff J. M., Langenberg A., Galloway D. A. Identification of immunoreactive antigens of human papillomavirus type 6b by using Escherichia coli-expressed fusion proteins. J Virol. 1988 Jun;62(6):2115–2123. doi: 10.1128/jvi.62.6.2115-2123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenison S. A., Yu X. P., Valentine J. M., Galloway D. A. Characterization of human antibody-reactive epitopes encoded by human papillomavirus types 16 and 18. J Virol. 1991 Mar;65(3):1208–1218. doi: 10.1128/jvi.65.3.1208-1218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio-Kokko H., Vapalahti O., Hedman K., Brummer-Korvenkontio M., Vaheri A. Puumala virus antibody and immunoglobulin G avidity assays based on a recombinant nucleocapsid antigen. J Clin Microbiol. 1993 Mar;31(3):677–680. doi: 10.1128/jcm.31.3.677-680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Lundkvist A., Fatouros A., Niklasson B. Antigenic variation of European haemorrhagic fever with renal syndrome virus strains characterized using bank vole monoclonal antibodies. J Gen Virol. 1991 Sep;72(Pt 9):2097–2103. doi: 10.1099/0022-1317-72-9-2097. [DOI] [PubMed] [Google Scholar]
- Lundkvist A., Niklasson B. Bank vole monoclonal antibodies against Puumala virus envelope glycoproteins: identification of epitopes involved in neutralization. Arch Virol. 1992;126(1-4):93–105. doi: 10.1007/BF01309687. [DOI] [PubMed] [Google Scholar]
- Nichol S. T., Spiropoulou C. F., Morzunov S., Rollin P. E., Ksiazek T. G., Feldmann H., Sanchez A., Childs J., Zaki S., Peters C. J. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993 Nov 5;262(5135):914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Parrington M. A., Kang C. Y. Nucleotide sequence analysis of the S genomic segment of Prospect Hill virus: comparison with the prototype Hantavirus. Virology. 1990 Mar;175(1):167–175. doi: 10.1016/0042-6822(90)90197-y. [DOI] [PubMed] [Google Scholar]
- Parrington M. A., Lee P. W., Kang C. Y. Molecular characterization of the Prospect Hill virus M RNA segment: a comparison with the M RNA segments of other hantaviruses. J Gen Virol. 1991 Aug;72(Pt 8):1845–1854. doi: 10.1099/0022-1317-72-8-1845. [DOI] [PubMed] [Google Scholar]
- Ruo S. L., Sanchez A., Elliott L. H., Brammer L. S., McCormick J. B., Fisher-Hoch S. P. Monoclonal antibodies to three strains of hantaviruses: Hantaan, R22, and Puumala. Arch Virol. 1991;119(1-2):1–11. doi: 10.1007/BF01314318. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C. S., Chu Y. K., Schmaljohn A. L., Dalrymple J. M. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J Virol. 1990 Jul;64(7):3162–3170. doi: 10.1128/jvi.64.7.3162-3170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C. S., Hasty S. E., Dalrymple J. M., LeDuc J. W., Lee H. W., von Bonsdorff C. H., Brummer-Korvenkontio M., Vaheri A., Tsai T. F., Regnery H. L. Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome. Science. 1985 Mar 1;227(4690):1041–1044. doi: 10.1126/science.2858126. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C. S., Jennings G. B., Hay J., Dalrymple J. M. Coding strategy of the S genome segment of Hantaan virus. Virology. 1986 Dec;155(2):633–643. doi: 10.1016/0042-6822(86)90223-0. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C. S., Schmaljohn A. L., Dalrymple J. M. Hantaan virus M RNA: coding strategy, nucleotide sequence, and gene order. Virology. 1987 Mar;157(1):31–39. doi: 10.1016/0042-6822(87)90310-2. [DOI] [PubMed] [Google Scholar]
- Vapalahti O., Kallio-Kokko H., Salonen E. M., Brummer-Korvenkontio M., Vaheri A. Cloning and sequencing of Puumala virus Sotkamo strain S and M RNA segments: evidence for strain variation in hantaviruses and expression of the nucleocapsid protein. J Gen Virol. 1992 Apr;73(Pt 4):829–838. doi: 10.1099/0022-1317-73-4-829. [DOI] [PubMed] [Google Scholar]
- Wang M., Pennock D. G., Spik K. W., Schmaljohn C. S. Epitope mapping studies with neutralizing and non-neutralizing monoclonal antibodies to the G1 and G2 envelope glycoproteins of Hantaan virus. Virology. 1993 Dec;197(2):757–766. doi: 10.1006/viro.1993.1652. [DOI] [PubMed] [Google Scholar]
- Wang M., Rossi C., Schmaljohn C. S. Expression of non-conserved regions of the S genome segments of three hantaviruses: evaluation of the expressed polypeptides for diagnosis of haemorrhagic fever with renal syndrome. J Gen Virol. 1993 Jun;74(Pt 6):1115–1124. doi: 10.1099/0022-1317-74-6-1115. [DOI] [PubMed] [Google Scholar]
- Xu X., Ruo S. L., McCormick J. B., Fisher-Hoch S. P. Immunity to Hantavirus challenge in Meriones unguiculatus induced by vaccinia-vectored viral proteins. Am J Trop Med Hyg. 1992 Oct;47(4):397–404. doi: 10.4269/ajtmh.1992.47.397. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu K., Yoo Y. C., Yoshida R., Ishihara C., Azuma I., Arikawa J. Protective immunity of Hantaan virus nucleocapsid and envelope protein studied using baculovirus-expressed proteins. Arch Virol. 1993;130(3-4):365–376. doi: 10.1007/BF01309667. [DOI] [PubMed] [Google Scholar]
- Zöller L., Yang S., Gött P., Bautz E. K., Darai G. Use of recombinant nucleocapsid proteins of the Hantaan and nephropathia epidemica serotypes of Hantaviruses as immunodiagnostic antigens. J Med Virol. 1993 Mar;39(3):200–207. doi: 10.1002/jmv.1890390305. [DOI] [PubMed] [Google Scholar]