Abstract
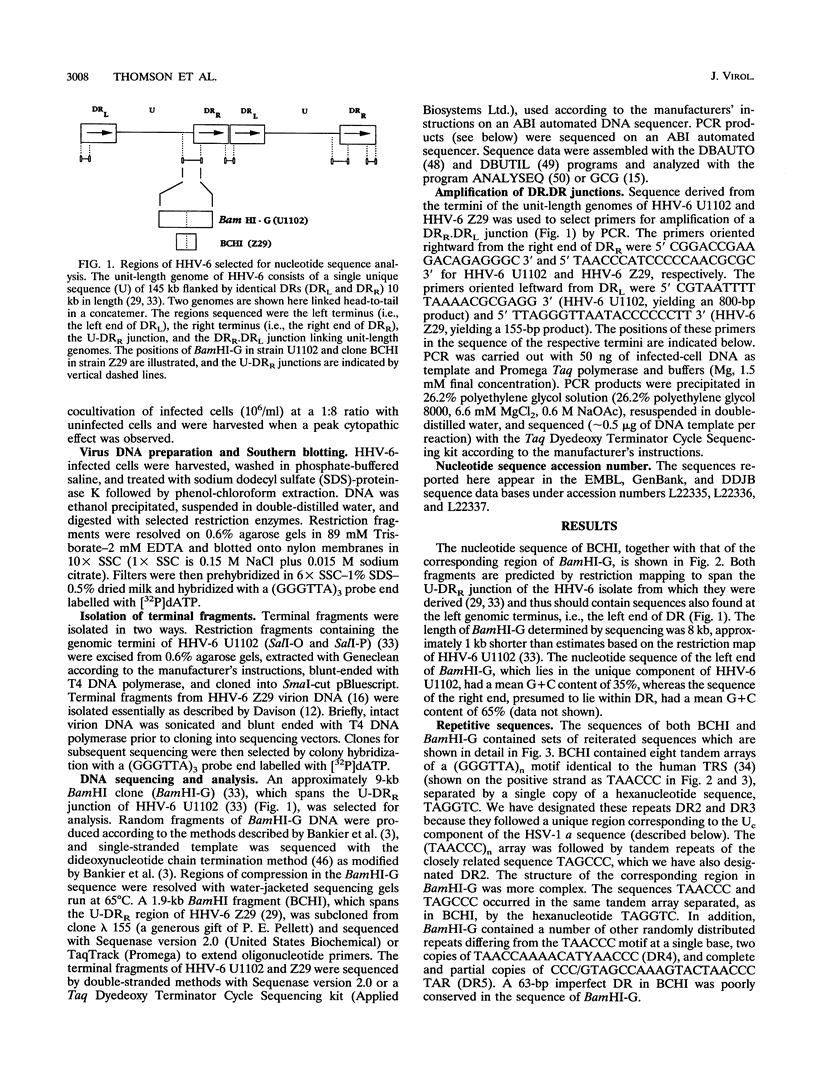
The unit-length genome of human herpesvirus 6 (HHV-6) consists of a single unique component (U) bounded by direct repeats DRL and DRR and forms head-to-tail concatemers during productive infection. cis-elements which mediate cleavage and packaging of progeny virions (a sequences) are found at the termini of all herpesvirus genomes. In HHV-6, DRL and DRR are identical and a sequences may therefore also occur at the U-DR junctions to give the arrangement aDRLa-U-aDRRa. We have sequenced the genomic termini, the U-DRR junction, and the DRR.DRL junction of HHV-6 strain variants U1102 and Z29. A (GGGTTA)n motif identical to the human telomeric repeat sequence (TRS) was found adjacent to, but did not form, the termini of both strain variants. The DRL terminus and U-DRR junction contained sequences closely related to that of the well-conserved herpesvirus packaging signal Cn-Gn-Nn-Gn (pac-1), followed by tandem arrays of TRSs separated by single copies of a hexanucleotide repeat. HHV-6 strain U1102 contained repeat sequences not found in HHV-6 Z29. In contrast, the DRR terminus of both variants contained a simple tandem array of TRSs and a close homolog of a herpesvirus pac-2 signal (GCn-Tn-GCn). The DRR.DRL junction was formed by simple head-to-tail linkage of the termini, yielding an intact cleavage signal, pac-2.x.pac-1, where x is the putative cleavage site. The left end of DR was the site of intrastrain size heterogeneity which mapped to the putative a sequences. These findings suggest that TRSs form part of the a sequence of HHV-6 and that the arrangement of a sequences in the genome can be represented as aDRLa-U-a-DRRa.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankier A. T., Dietrich W., Baer R., Barrell B. G., Colbère-Garapin F., Fleckenstein B., Bodemer W. Terminal repetitive sequences in herpesvirus saimiri virion DNA. J Virol. 1985 Jul;55(1):133–139. doi: 10.1128/jvi.55.1.133-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Brown W. R., MacKinnon P. J., Villasanté A., Spurr N., Buckle V. J., Dobson M. J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990 Oct 5;63(1):119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Browning G. F., Studdert M. J. Physical mapping of a genome of equine herpesvirus 2 (equine cytomegalovirus). Arch Virol. 1989;104(1-2):77–86. doi: 10.1007/BF01313809. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Knox K. K., Tapper M. A. Suppression of human immunodeficiency virus type 1 replication by human herpesvirus-6. J Infect Dis. 1990 Oct;162(4):844–851. doi: 10.1093/infdis/162.4.844. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Balachandran N. Identification, characterization, and sequence analysis of a cDNA encoding a phosphoprotein of human herpesvirus 6. J Virol. 1991 Jun;65(6):2884–2894. doi: 10.1128/jvi.65.6.2884-2894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman S., Lacasa M., Sheldrick P. Physical Map of the Channel Catfish Virus Genome: Location of Sites for Restriction Endonucleases EcoRI, HindIII, HpaI, and XbaI. J Virol. 1979 Jul;31(1):73–85. doi: 10.1128/jvi.31.1.73-85.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J. Channel catfish virus: a new type of herpesvirus. Virology. 1992 Jan;186(1):9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Structure of the genome termini of varicella-zoster virus. J Gen Virol. 1984 Nov;65(Pt 11):1969–1977. doi: 10.1099/0022-1317-65-11-1969. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981 Aug;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Deiss L. P., Chou J., Frenkel N. Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J Virol. 1986 Sep;59(3):605–618. doi: 10.1128/jvi.59.3.605-618.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S., Chandran B., McIntyre K., Schnabel K., Hall C. B. Phenotypic and genetic polymorphisms among human herpesvirus-6 isolates from North American infants. Virology. 1992 Sep;190(1):490–493. doi: 10.1016/0042-6822(92)91240-u. [DOI] [PubMed] [Google Scholar]
- Downing R. G., Sewankambo N., Serwadda D., Honess R., Crawford D., Jarrett R., Griffin B. E. Isolation of human lymphotropic herpesviruses from Uganda. Lancet. 1987 Aug 15;2(8555):390–390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Lawrence G. L., Brown C. M., Barrell B. G. Identification of homologues to the human cytomegalovirus US22 gene family in human herpesvirus 6. J Gen Virol. 1992 Jul;73(Pt 7):1661–1671. doi: 10.1099/0022-1317-73-7-1661. [DOI] [PubMed] [Google Scholar]
- Ellinger K., Neipel F., Foà-Tomasi L., Campadelli-Fiume G., Fleckenstein B. The glycoprotein B homologue of human herpesvirus 6. J Gen Virol. 1993 Mar;74(Pt 3):495–500. doi: 10.1099/0022-1317-74-3-495. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Carss A. L., Sun N., Arrand J. R. Infectivity determinants encoded in a conserved gene block of human herpesvirus-6. DNA Seq. 1992;3(1):25–39. doi: 10.3109/10425179209039693. [DOI] [PubMed] [Google Scholar]
- Gopal M. R., Thomson B. J., Fox J., Tedder R. S., Honess R. W. Detection by PCR of HHV-6 and EBV DNA in blood and oropharynx of healthy adults and HIV-seropositives. Lancet. 1990 Jun 30;335(8705):1598–1599. doi: 10.1016/0140-6736(90)91433-b. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Ludwig H., Buhk H. J. Short repeats cause heterogeneity at genomic terminus of bovine herpesvirus 1. J Virol. 1986 Apr;58(1):43–49. doi: 10.1128/jvi.58.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human herpesvirus-6 strain groups: a nomenclature. Arch Virol. 1993;129(1-4):363–366. doi: 10.1007/BF01316913. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Ablashi D. V., Salahuddin S. Z., Jagodzinski L. L., Wong-Staal F., Gallo R. C. Identification of the human herpesvirus 6 glycoprotein H and putative large tegument protein genes. J Virol. 1991 Oct;65(10):5597–5604. doi: 10.1128/jvi.65.10.5597-5604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M., Bradley G., Jessip J., Tanaka A., Nonoyama M. Inverted repeat regions of Marek's disease virus DNA possess a structure similar to that of the a sequence of herpes simplex virus DNA and contain host cell telomere sequences. J Virol. 1991 Jun;65(6):2791–2797. doi: 10.1128/jvi.65.6.2791-2797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M., Harada H., Takahashi M., Tanaka A., Hayashi M., Nonoyama M., Josephs S. F., Buchbinder A., Schachter F., Ablashi D. V. A repeat sequence, GGGTTA, is shared by DNA of human herpesvirus 6 and Marek's disease virus. J Virol. 1988 Dec;62(12):4824–4827. doi: 10.1128/jvi.62.12.4824-4827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Kondo T., Okuno T., Takahashi M., Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991 Jun;72(Pt 6):1401–1408. doi: 10.1099/0022-1317-72-6-1401. [DOI] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquester G. J., Pellett P. E. Properties of the human herpesvirus 6 strain Z29 genome: G + C content, length, and presence of variable-length directly repeated terminal sequence elements. Virology. 1991 May;182(1):102–110. doi: 10.1016/0042-6822(91)90653-s. [DOI] [PubMed] [Google Scholar]
- Longtine M. S., Enomoto S., Finstad S. L., Berman J. Yeast telomere repeat sequence (TRS) improves circular plasmid segregation, and TRS plasmid segregation involves the RAP1 gene product. Mol Cell Biol. 1992 May;12(5):1997–2009. doi: 10.1128/mcb.12.5.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., Pellett P., Stewart J., Goldsmith C., Sanderlin K., Black J., Warfield D., Feorino P. Characteristics of human herpesvirus-6. J Infect Dis. 1988 Jun;157(6):1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- Martin M. E., Nicholas J., Thomson B. J., Newman C., Honess R. W. Identification of a transactivating function mapping to the putative immediate-early locus of human herpesvirus 6. J Virol. 1991 Oct;65(10):5381–5390. doi: 10.1128/jvi.65.10.5381-5390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Thomson B. J., Honess R. W., Craxton M. A., Gompels U. A., Liu M. Y., Littler E., Arrand J. R., Teo I., Jones M. D. The genome of human herpesvirus 6: maps of unit-length and concatemeric genomes for nine restriction endonucleases. J Gen Virol. 1991 Jan;72(Pt 1):157–168. doi: 10.1099/0022-1317-72-1-157. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Liu A. C., Spaete R. R. Structure and variability of the a sequence in the genome of human cytomegalovirus (Towne strain). J Gen Virol. 1987 Aug;68(Pt 8):2223–2230. doi: 10.1099/0022-1317-68-8-2223. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Site-specific inversion sequence of the herpes simplex virus genome: domain and structural features. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7047–7051. doi: 10.1073/pnas.78.11.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell. 1982 Nov;31(1):89–97. doi: 10.1016/0092-8674(82)90408-1. [DOI] [PubMed] [Google Scholar]
- Neipel F., Ellinger K., Fleckenstein B. The unique region of the human herpesvirus 6 genome is essentially collinear with the UL segment of human cytomegalovirus. J Gen Virol. 1991 Sep;72(Pt 9):2293–2297. doi: 10.1099/0022-1317-72-9-2293. [DOI] [PubMed] [Google Scholar]
- Okuno T., Higashi K., Shiraki K., Yamanishi K., Takahashi M., Kokado Y., Ishibashi M., Takahara S., Sonoda T., Tanaka K. Human herpesvirus 6 infection in renal transplantation. Transplantation. 1990 Mar;49(3):519–522. doi: 10.1097/00007890-199003000-00009. [DOI] [PubMed] [Google Scholar]
- Okuno T., Takahashi K., Balachandra K., Shiraki K., Yamanishi K., Takahashi M., Baba K. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989 Apr;27(4):651–653. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesano R. L., Pagano J. S. Herpesvirus papio contains a plasmid origin of replication that acts in cis interspecies with an Epstein-Barr virus trans-acting function. J Virol. 1986 Dec;60(3):1159–1162. doi: 10.1128/jvi.60.3.1159-1162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. D., Silva R. F. The number of copies of an a-like region in the serotype-3 Marek's disease virus DNA genome is variable. Virology. 1993 Mar;193(1):268–280. doi: 10.1006/viro.1993.1122. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc Natl Acad Sci U S A. 1985 Feb;82(3):694–698. doi: 10.1073/pnas.82.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Measurements of the effects that coding for a protein has on a DNA sequence and their use for finding genes. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):551–567. doi: 10.1093/nar/12.1part2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., McMonagle E. C., Davison A. J. Fragments from both termini of the herpes simplex virus type 1 genome contain signals required for the encapsidation of viral DNA. Nucleic Acids Res. 1983 Dec 10;11(23):8205–8220. doi: 10.1093/nar/11.23.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Filpula D., Friedmann T., Spector D. H. Structure of the heterogeneous L-S junction region of human cytomegalovirus strain AD169 DNA. J Virol. 1984 Nov;52(2):541–548. doi: 10.1128/jvi.52.2.541-548.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Spector D. H. Terminal structure and heterogeneity in human cytomegalovirus strain AD169. J Virol. 1986 Sep;59(3):591–604. doi: 10.1128/jvi.59.3.591-604.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder R. S., Briggs M., Cameron C. H., Honess R., Robertson D., Whittle H. A novel lymphotropic herpesvirus. Lancet. 1987 Aug 15;2(8555):390–392. doi: 10.1016/s0140-6736(87)92404-4. [DOI] [PubMed] [Google Scholar]
- Teo I. A., Griffin B. E., Jones M. D. Characterization of the DNA polymerase gene of human herpesvirus 6. J Virol. 1991 Sep;65(9):4670–4680. doi: 10.1128/jvi.65.9.4670-4680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson B. J., Honess R. W. The right end of the unique region of the genome of human herpesvirus 6 U1102 contains a candidate immediate early gene enhancer and a homologue of the human cytomegalovirus US22 gene family. J Gen Virol. 1992 Jul;73(Pt 7):1649–1660. doi: 10.1099/0022-1317-73-7-1649. [DOI] [PubMed] [Google Scholar]
- Varmuza S. L., Smiley J. R. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell. 1985 Jul;41(3):793–802. doi: 10.1016/s0092-8674(85)80060-x. [DOI] [PubMed] [Google Scholar]
- Vlazny D. A., Frenkel N. Replication of herpes simplex virus DNA: localization of replication recognition signals within defective virus genomes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):742–746. doi: 10.1073/pnas.78.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K. N., Gray J. J., Efstathiou S. Brief report: primary human herpesvirus 6 infection in a patient following liver transplantation from a seropositive donor. J Med Virol. 1989 Jun;28(2):69–72. doi: 10.1002/jmv.1890280203. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]