Abstract
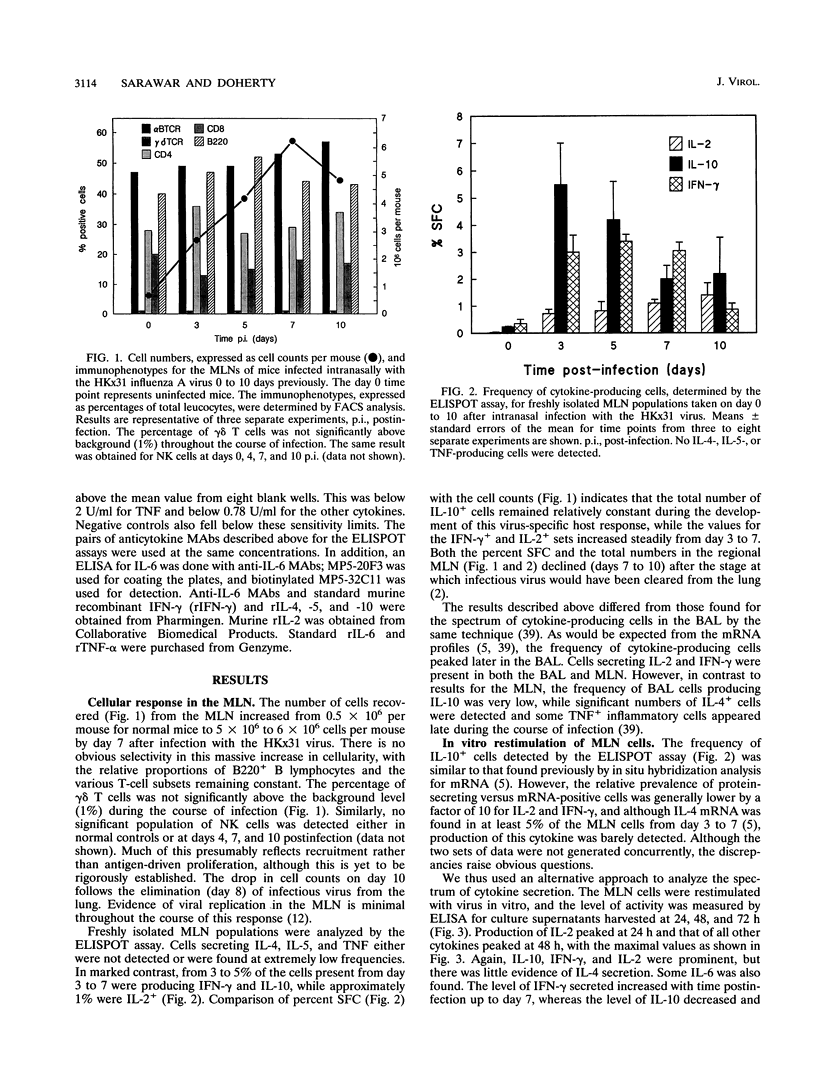
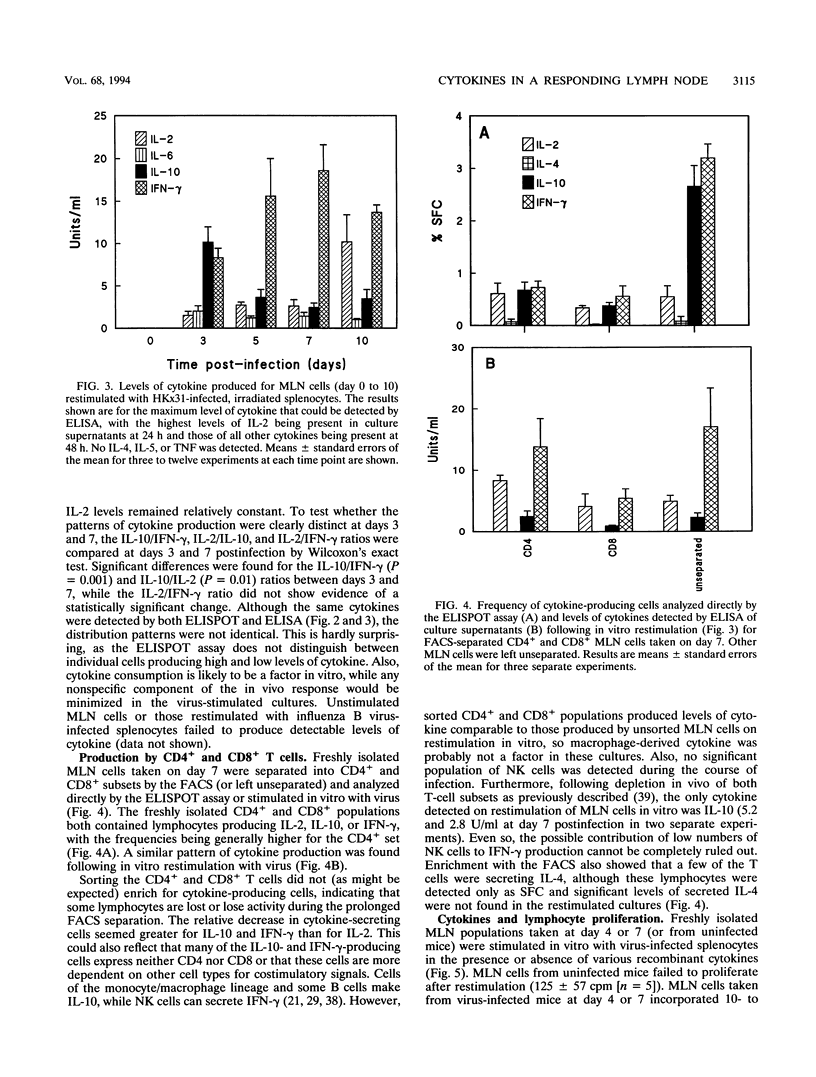
Cytokine production has been assessed at the single-cell level (ELISPOT assay) for freshly isolated mediastinal lymph node cells from C57BL/6 mice with primary, nonfatal influenza pneumonia. The mediastinal lymph node populations were also secondarily stimulated in vitro, and culture supernatants were assayed by enzyme-linked immunosorbent assay. Both approaches showed minimal evidence of protein secretion for interleukin-4 (IL-4), IL-5, and tumor necrosis factor, while IL-2, IL-10, and gamma interferon (IFN-gamma) were prominent throughout the response. The numbers of IL-2- and IFN-gamma-producing cells were maximal at 7 days after infection, while the total counts for cells secreting IL-10 were fairly constant from day 3 to 7. The cultures that were stimulated with virus in vitro showed in inverse relationship between IL-10 and IFN-gamma production, with IL-10 peaking on day 3 and IFN-gamma peaking on day 7. Lymphocytes secreting IL-2, IL-10, and/or IFN-gamma were present in CD4+ and CD8+ populations separated by fluorescence-activated cell sorting, although the CD8+ T cells produced less cytokine and were at a relatively lower frequency. Addition of recombinant IL-10 to the virus-stimulated cultures decreased the amount of IFN-gamma that could be detected, while incorporation of a monoclonal antibody to IL-10 had the opposite effect. A neutralization experiment also indicated that IL-2 was the principal mediator of lymphocyte proliferation. These experiments thus show that the developing T-cell response in the regional lymph nodes of mice with influenza cannot be rigidly categorized on the basis of a TH1 or TH2 phenotype and suggest possible regulatory mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Jones P. D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- Allan W., Tabi Z., Cleary A., Doherty P. C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990 May 15;144(10):3980–3986. [PubMed] [Google Scholar]
- Balkovic E. S., Florack J. A., Six H. R. Immunoglobulin G subclass antibody responses of mice to influenza virus antigens given in different forms. Antiviral Res. 1987 Oct;8(3):151–160. doi: 10.1016/0166-3542(87)90068-4. [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Duncan D. D., Tonkonogy S., Swain S. L. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J Exp Med. 1991 Sep 1;174(3):547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carding S. R., Allan W., McMickle A., Doherty P. C. Activation of cytokine genes in T cells during primary and secondary murine influenza pneumonia. J Exp Med. 1993 Feb 1;177(2):475–482. doi: 10.1084/jem.177.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. F., Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991 Jul 15;147(2):528–534. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Vink A., van Snick J. Virally induced modulation of murine IgG antibody subclasses. J Exp Med. 1988 Dec 1;168(6):2373–2378. doi: 10.1084/jem.168.6.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Interferon-gamma. Curr Opin Immunol. 1992 Jun;4(3):321–326. doi: 10.1016/0952-7915(92)90083-q. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberger M. C., Wang M. L., Allan W., Webster R. G., Doherty P. C. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991 Jul;72(Pt 7):1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Fujihashi K., McGhee J. R., Beagley K. W., McPherson D. T., McPherson S. A., Huang C. M., Kiyono H. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4 and IL-6 producing cells. J Immunol Methods. 1993 Apr 2;160(2):181–189. doi: 10.1016/0022-1759(93)90176-8. [DOI] [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L. Production and characterization of anti-murine interferon-gamma sera. J Interferon Res. 1983;3(2):191–198. doi: 10.1089/jir.1983.3.191. [DOI] [PubMed] [Google Scholar]
- Hennet T., Ziltener H. J., Frei K., Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992 Aug 1;149(3):932–939. [PubMed] [Google Scholar]
- Hilbert D. M., Cancro M. P., Scherle P. A., Nordan R. P., Van Snick J., Gerhard W., Rudikoff S. T cell derived IL-6 is differentially required for antigen-specific antibody secretion by primary and secondary B cells. J Immunol. 1989 Dec 15;143(12):4019–4024. [PubMed] [Google Scholar]
- Hou S., Doherty P. C. Partitioning of responder CD8+ T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD45RB phenotype. J Immunol. 1993 Jun 15;150(12):5494–5500. [PubMed] [Google Scholar]
- Hou S., Doherty P. C., Zijlstra M., Jaenisch R., Katz J. M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992 Aug 15;149(4):1319–1325. [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41(3):643–645. [PMC free article] [PubMed] [Google Scholar]
- Kos F. J., Müllbacher A. Induction of primary anti-viral cytotoxic T cells by in vitro stimulation with short synthetic peptide and interleukin-7. Eur J Immunol. 1992 Dec;22(12):3183–3185. doi: 10.1002/eji.1830221224. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989 Mar 31;243(4899):1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- Lynch F., Doherty P. C., Ceredig R. Phenotypic and functional analysis of the cellular response in regional lymphoid tissue during an acute virus infection. J Immunol. 1989 May 15;142(10):3592–3598. [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Michael A., Hackett J. J., Bennett M., Kumar V., Yuan D. Regulation of B lymphocytes by natural killer cells. Role of IFN-gamma. J Immunol. 1989 Feb 15;142(4):1095–1101. [PubMed] [Google Scholar]
- Miller R. A., Reiss C. S. Limiting dilution cultures reveal latent influenza virus-specific helper T cells in virus-primed mice. J Mol Cell Immunol. 1984;1(6):357–368. [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Hämmerling G. J. In vivo induction of H-2K/D antigens by recombinant interferon-gamma. Eur J Immunol. 1986 May;16(5):551–557. doi: 10.1002/eji.1830160516. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Ochiai H., Ikesue A., Kurokawa M., Nakajima K., Nakagawa H. Enhanced production of rat interleukin-8 by in vitro and in vivo infections with influenza A NWS virus. J Virol. 1993 Nov;67(11):6811–6814. doi: 10.1128/jvi.67.11.6811-6814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Quesniaux V. F. Interleukins 9, 10, 11 and 12 and kit ligand: a brief overview. Res Immunol. 1992 May;143(4):385–400. doi: 10.1016/s0923-2494(05)80071-9. [DOI] [PubMed] [Google Scholar]
- Sandvig S., Laskay T., Andersson J., De Ley M., Andersson U. Gamma-interferon is produced by CD3+ and CD3- lymphocytes. Immunol Rev. 1987 Jun;97:51–65. doi: 10.1111/j.1600-065x.1987.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Sarawar S. R., Carding S. R., Allan W., McMickle A., Fujihashi K., Kiyono H., McGhee J. R., Doherty P. C. Cytokine profiles of bronchoalveolar lavage cells from mice with influenza pneumonia: consequences of CD4+ and CD8+ T cell depletion. Reg Immunol. 1993 May-Aug;5(3-4):142–150. [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2. Curr Opin Immunol. 1992 Jun;4(3):271–276. doi: 10.1016/0952-7915(92)90076-q. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Mond J. J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993 Jan;14(1):15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Meager A., Askonas B. A. Influenza virus-specific T cells lead to early interferon gamma in lungs of infected hosts: development of a sensitive radioimmunoassay. J Gen Virol. 1989 Apr;70(Pt 4):975–978. doi: 10.1099/0022-1317-70-4-975. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993 Jul;14(7):335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- Vacheron F., Rudent A., Perin S., Labarre C., Quero A. M., Guenounou M. Production of interleukin 1 and tumour necrosis factor activities in bronchoalveolar washings following infection of mice by influenza virus. J Gen Virol. 1990 Feb;71(Pt 2):477–479. doi: 10.1099/0022-1317-71-2-477. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., Harris A. W., Schrader J. W. Effect of cloned interferon-gamma on expression of H-2 and Ia antigens on cell lines of hemopoietic, lymphoid, epithelial, fibroblastic and neuronal origin. Eur J Immunol. 1984 Jan;14(1):52–56. doi: 10.1002/eji.1830140110. [DOI] [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]



