Abstract
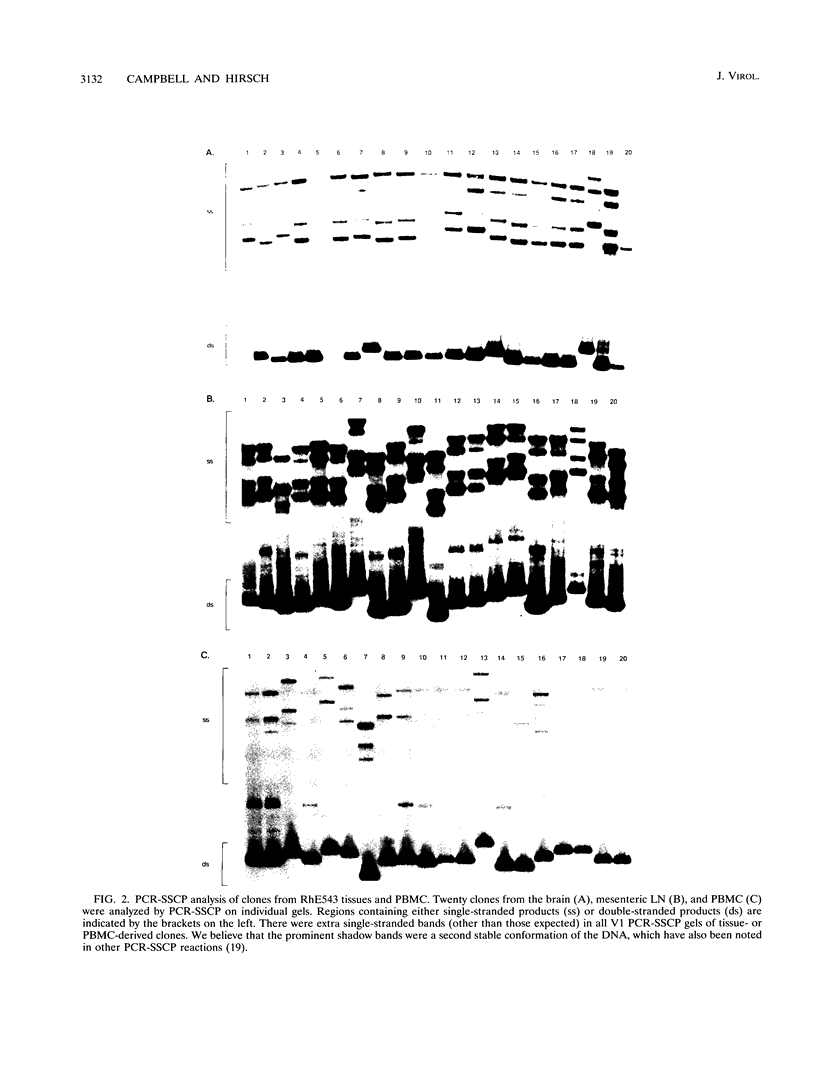
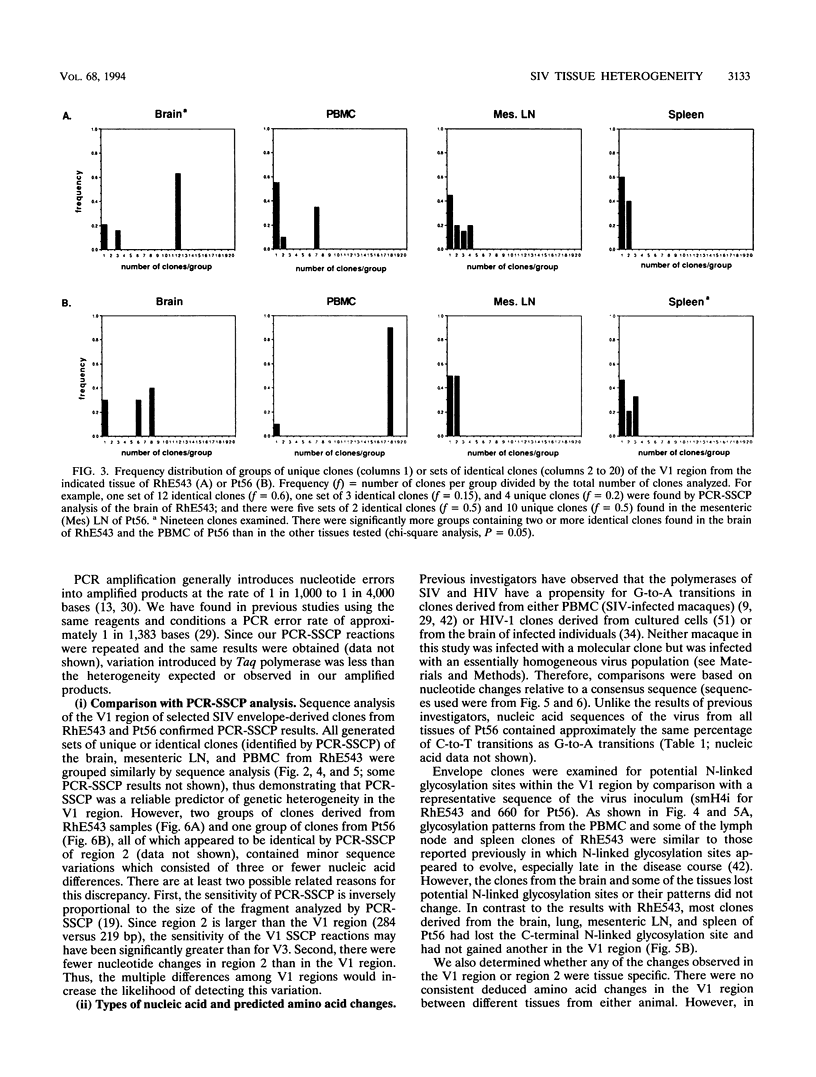
The extent of virus genetic variation within tissues and peripheral blood mononuclear cells (PBMC) from two simian immunodeficiency virus (SIV)-infected macaques was analyzed. The products of PCR amplification of two regions, region 1 (SIV V1 region) and region 2 (region corresponding to the human immunodeficiency virus V3 cysteine loop and part of the C3 region immediately downstream), of the SIV envelope were examined for single-stranded conformation polymorphism followed by sequence analysis of selected clones. The V1 region of the SIV envelope of viruses present within lymphoid tissues displayed extensive heterogeneity, while viral populations within the PBMC and brain appeared to be less variable. Region 2 heterogeneity in both animals was generally confined to three residues in a tissue-specific manner. In addition, virus from the brains of both animals appeared to be distinct compared with viruses present in other tissues and PBMC of the same animal, both in the pattern of PCR-single-stranded conformation polymorphism SCP and in the sequence of region 2. These studies revealed that the tissues of SIV-infected macaques were a reservoir for viral variants distinct from those seen in PBMC.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Abrahamsson B., Nagy K., Aurelius E., Gaines H., Nyström G., Fenyö E. M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990 Feb;4(2):107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Almond N., Jenkins A., Heath A. B., Kitchin P. Sequence variation in the env gene of simian immunodeficiency virus recovered from immunized macaques is predominantly in the V1 region. J Gen Virol. 1993 May;74(Pt 5):865–871. doi: 10.1099/0022-1317-74-5-865. [DOI] [PubMed] [Google Scholar]
- Almond N., Jenkins A., Slade A., Heath A., Cranage M., Kitchin P. Population sequence analysis of a simian immunodeficiency virus (32H reisolate of SIVmac251): a virus stock used for international vaccine studies. AIDS Res Hum Retroviruses. 1992 Jan;8(1):77–88. doi: 10.1089/aid.1992.8.77. [DOI] [PubMed] [Google Scholar]
- Anderson M. G., Hauer D., Sharma D. P., Joag S. V., Narayan O., Zink M. C., Clements J. E. Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology. 1993 Aug;195(2):616–626. doi: 10.1006/viro.1993.1413. [DOI] [PubMed] [Google Scholar]
- Biebricher C. K., Eigen M., Gardiner W. C., Jr Kinetics of RNA replication: competition and selection among self-replicating RNA species. Biochemistry. 1985 Nov 5;24(23):6550–6560. doi: 10.1021/bi00344a037. [DOI] [PubMed] [Google Scholar]
- Boeri E., Giri A., Lillo F., Ferrari G., Varnier O. E., Ferro A., Sabbatani S., Saxinger W. C., Franchini G. In vivo genetic variability of the human immunodeficiency virus type 2 V3 region. J Virol. 1992 Jul;66(7):4546–4550. doi: 10.1128/jvi.66.7.4546-4550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. P., Collignon C., Desrosiers R. C. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J Virol. 1993 Jul;67(7):4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. P., Desrosiers R. C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991 Apr;65(4):1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Ennis P. D., Zemmour J., Salter R. D., Parham P. Rapid cloning of HLA-A,B cDNA by using the polymerase chain reaction: frequency and nature of errors produced in amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2833–2837. doi: 10.1073/pnas.87.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. G., Kuiken C., Blumberg B. M., Hartman S., Sharer L. R., Clement M., Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991 Feb;180(2):583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- Goodenow M., Huet T., Saurin W., Kwok S., Sninsky J., Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J Acquir Immune Defic Syndr. 1989;2(4):344–352. [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Hayashi K. PCR-SSCP: a method for detection of mutations. Genet Anal Tech Appl. 1992 Jun;9(3):73–79. doi: 10.1016/1050-3862(92)90001-l. [DOI] [PubMed] [Google Scholar]
- Hayashi K. PCR-SSCP: a simple and sensitive method for detection of mutations in the genomic DNA. PCR Methods Appl. 1991 Aug;1(1):34–38. doi: 10.1101/gr.1.1.34. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hirsch V. M., Dapolito G., McGann C., Olmsted R. A., Purcell R. H., Johnson P. R. Molecular cloning of SIV from sooty mangabey monkeys. J Med Primatol. 1989;18(3-4):279–285. [PubMed] [Google Scholar]
- Hirsch V. M., Martin J. E., Dapolito G., Elkins W. R., London W. T., Goldstein S., Johnson P. R. Spontaneous substitutions in the vicinity of the V3 analog affect cell tropism and pathogenicity of simian immunodeficiency virus. J Virol. 1994 Apr;68(4):2649–2661. doi: 10.1128/jvi.68.4.2649-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Olmsted R. A., Murphey-Corb M., Purcell R. H., Johnson P. R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989 Jun 1;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V. M., Zack P. M., Vogel A. P., Johnson P. R. Simian immunodeficiency virus infection of macaques: end-stage disease is characterized by widespread distribution of proviral DNA in tissues. J Infect Dis. 1991 May;163(5):976–988. doi: 10.1093/infdis/163.5.976. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Hynes N. A., Adger-Johnson D., Dapolito G., Hirsch V. M. Rapid screening for simian immunodeficiency virus variants using single-strand conformation polymorphism of PCR-amplified DNA fragments. AIDS Res Hum Retroviruses. 1993 Aug;9(8):803–806. doi: 10.1089/aid.1993.9.803. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., Schmidt S., Kaufmann M., Cates N., Langedijk J. P., Meloen R. H., Desrosiers R. C., Burns D. P., Bolognesi D. P. The principal neutralization determinant of simian immunodeficiency virus differs from that of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1418–1422. doi: 10.1073/pnas.89.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Hamm T. E., Goldstein S., Kitov S., Hirsch V. M. The genetic fate of molecularly cloned simian immunodeficiency virus in experimentally infected macaques. Virology. 1991 Nov;185(1):217–228. doi: 10.1016/0042-6822(91)90769-8. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Burns D. P., Silva D. P., Veronese F. D., Desrosiers R. C. Strain-specific neutralizing determinant in the transmembrane protein of simian immunodeficiency virus. J Virol. 1991 Apr;65(4):2010–2018. doi: 10.1128/jvi.65.4.2010-2018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Mori K., Kawahara T., Ringler D. J., Desrosiers R. C. Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J Virol. 1993 Nov;67(11):6522–6534. doi: 10.1128/jvi.67.11.6522-6534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., King N. W. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3(11):1023–1040. [PubMed] [Google Scholar]
- Li Y., Hui H., Burgess C. J., Price R. W., Sharp P. M., Hahn B. H., Shaw G. M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992 Nov;66(11):6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating J. A., Gow J., Goudsmit J., Pearl L. H., Mulder C., Weiss R. A. Characterization of HIV-1 neutralization escape mutants. AIDS. 1989 Dec;3(12):777–784. doi: 10.1097/00002030-198912000-00001. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Desrosiers R. C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993 May;67(5):2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Kodama T., Desrosiers R. C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992 Apr;66(4):2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Smit L., Dunlop N., Hatch W., Merges M., Waters D., Kelliher J., Gallo R. C., Fischinger P. J., Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990 Aug;64(8):3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M. Alterations in potential sites for glycosylation predominate during evolution of the simian immunodeficiency virus envelope gene in macaques. J Virol. 1992 Oct;66(10):5937–5948. doi: 10.1128/jvi.66.10.5937-5948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M., Papenhausen M. D., Benveniste R. E., Morton W. R. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991 Dec;65(12):7025–7031. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Vinters H. V., Akashi T., O'Brien W. A., Chen I. S. HIV-1 env sequence variation in brain tissue of patients with AIDS-related neurologic disease. J Acquir Immune Defic Syndr. 1991;4(11):1082–1092. [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell-line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. A., Chalifoux L. V., Ringler D. J. Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res Hum Retroviruses. 1992 Mar;8(3):327–337. doi: 10.1089/aid.1992.8.327. [DOI] [PubMed] [Google Scholar]
- Tremblay M., Numazaki K., Li X. G., Gornitsky M., Hiscott J., Wainberg M. A. Resistance to infection by HIV-1 of peripheral blood mononuclear cells from HIV-1-infected patients is probably mediated by neutralizing antibodies. J Immunol. 1990 Nov 1;145(9):2896–2901. [PubMed] [Google Scholar]
- Tremblay M., Wainberg M. A. Neutralization of multiple HIV-1 isolates from a single subject by autologous sequential sera. J Infect Dis. 1990 Sep;162(3):735–737. doi: 10.1093/infdis/162.3.735. [DOI] [PubMed] [Google Scholar]
- Vartanian J. P., Meyerhans A., Asjö B., Wain-Hobson S. Selection, recombination, and G----A hypermutation of human immunodeficiency virus type 1 genomes. J Virol. 1991 Apr;65(4):1779–1788. doi: 10.1128/jvi.65.4.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tijn D. A., Boucher C. A., Bakker M., Goudsmit J. Antigenicity of linear B-cell epitopes in the C1, V1, and V3 region of HIV-1 gp 120. J Acquir Immune Defic Syndr. 1989;2(3):303–306. [PubMed] [Google Scholar]





