Abstract
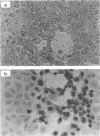
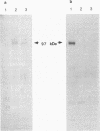
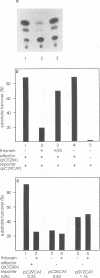
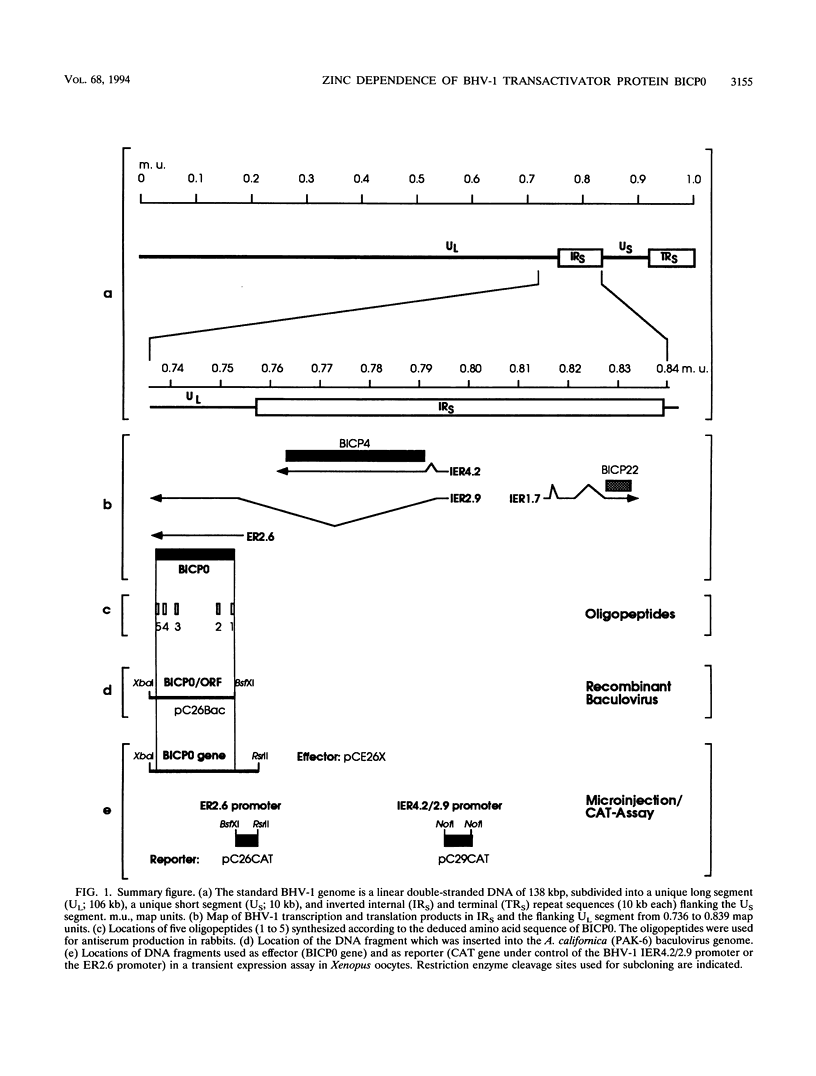
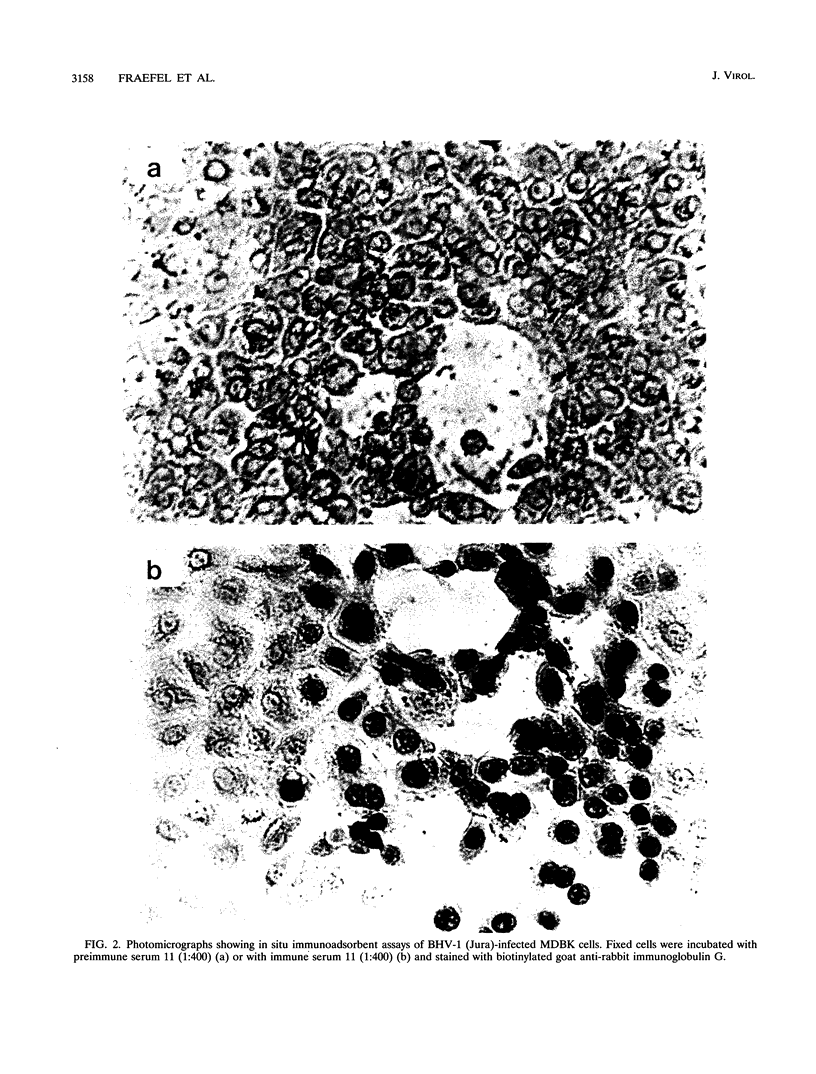
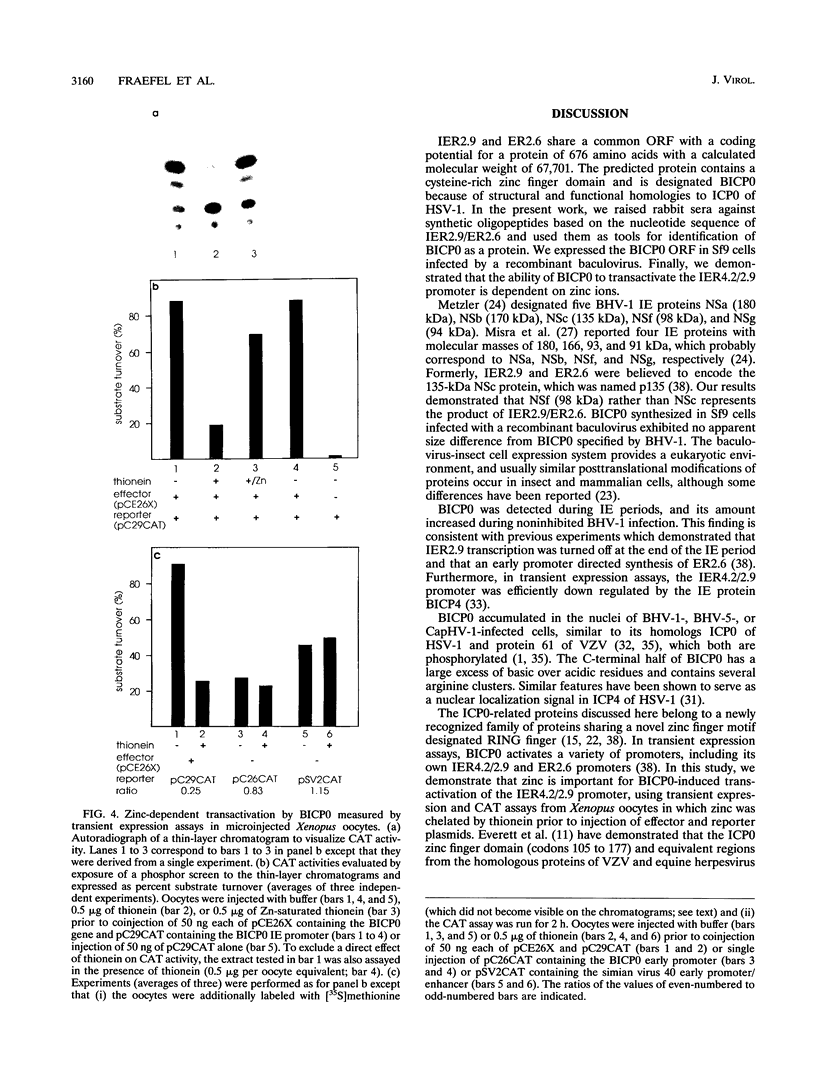
Bovine herpesvirus 1 (BHV-1) specifies and unspliced early 2.6-kb RNA (ER2.6) which is 3' coterminal with exon 2 of the 2.9-kb immediate-early (IE) RNA. The two transcripts have a common open reading frame (676 codons). The predicted protein, designated BHV-1 infected cell protein 0 (BICP0), contains a zinc finger domain with homology to ICP0 of herpes simplex virus type 1 and protein 61 of varicella-zoster virus, and depending on the promoter, it acts as a strong activator or as a repressor in transient expression assays. In situ immunoadsorbent assays using antisera against synthetic oligopeptides demonstrated that BICP0 accumulates in nuclei of BHV-1-infected cells, as expected for an IE gene product involved in gene regulation. Western blots (immunoblots) revealed a BHV-1-specific 97-kDa protein which was detectable during the IE phase and also at later periods of infection, indicating that the kinetics of BICP0 synthesis is consistent with the switch from IER2.9 to ER2.6. To confirm that ER2.6 encoded the 97-kDa BICP0 protein, a DNA fragment containing BICP0-coding sequences was inserted into the Autographa californica baculovirus genome. A recombinant protein, identified by its reactivity with antipeptide sera, exhibited the same electrophoretic mobility as BICP0 specified by BHV-1. We microinjected Xenopus oocytes with a BICP0 effector plasmid and a promoter-chloramphenicol acetyltransferase plasmid. BICP0-induced stimulation of this promoter was strongly reduced when intracellular zinc was chelated by thionein, indicating that the effect of BICP0 is zinc dependent.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Braun D. K., Pereira L., Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984 Oct;52(1):108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Schaffer P. A. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol. 1992 May;66(5):2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. X., Zhu X. X., Silverstein S. Mutational analysis of the sequence encoding ICP0 from herpes simplex virus type 1. Virology. 1991 Jan;180(1):207–220. doi: 10.1016/0042-6822(91)90025-7. [DOI] [PubMed] [Google Scholar]
- Chen J., Silverstein S. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J Virol. 1992 May;66(5):2916–2927. doi: 10.1128/jvi.66.5.2916-2927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels M., Loepfe E., Wild P., Schraner E., Wyler R. The genome of caprine herpesvirus 1: genome structure and relatedness to bovine herpesvirus 1. J Gen Virol. 1987 Jul;68(Pt 7):2019–2023. doi: 10.1099/0022-1317-68-7-2019. [DOI] [PubMed] [Google Scholar]
- Everett R. D. A detailed mutational analysis of Vmw110, a trans-acting transcriptional activator encoded by herpes simplex virus type 1. EMBO J. 1987 Jul;6(7):2069–2076. doi: 10.1002/j.1460-2075.1987.tb02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J Mol Biol. 1988 Jul 5;202(1):87–96. doi: 10.1016/0022-2836(88)90521-9. [DOI] [PubMed] [Google Scholar]
- Everett R. D., Barlow P., Milner A., Luisi B., Orr A., Hope G., Lyon D. A novel arrangement of zinc-binding residues and secondary structure in the C3HC4 motif of an alpha herpes virus protein family. J Mol Biol. 1993 Dec 20;234(4):1038–1047. doi: 10.1006/jmbi.1993.1657. [DOI] [PubMed] [Google Scholar]
- Everett R. D. Construction and characterization of herpes simplex type 1 viruses without introns in immediate early gene 1. J Gen Virol. 1991 Mar;72(Pt 3):651–659. doi: 10.1099/0022-1317-72-3-651. [DOI] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraefel C., Wirth U. V., Vogt B., Schwyzer M. Immediate-early transcription over covalently joined genome ends of bovine herpesvirus 1: the circ gene. J Virol. 1993 Mar;67(3):1328–1333. doi: 10.1128/jvi.67.3.1328-1333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Freemont P. S., Hanson I. M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991 Feb 8;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Friedli K., Metzler A. E. Reactivity of monoclonal antibodies to proteins of a neurotropic bovine herpesvirus 1 (BHV-1) strain and to proteins of representative BHV-1 strains. Arch Virol. 1987;94(1-2):109–122. doi: 10.1007/BF01313729. [DOI] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- KENDRICK J. W., GILLESPIE J. H., MCENTEE K. Infectious pustular vulvovaginitis of cattle. Cornell Vet. 1958 Oct;48(4):458–495. [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kousoulas K. G., Pellett P. E., Pereira L., Roizman B. Mutations affecting conformation or sequence of neutralizing epitopes identified by reactivity of viable plaques segregate from syn and ts domains of HSV-1(F) gB gene. Virology. 1984 Jun;135(2):379–394. doi: 10.1016/0042-6822(84)90194-6. [DOI] [PubMed] [Google Scholar]
- Lovering R., Hanson I. M., Borden K. L., Martin S., O'Reilly N. J., Evan G. I., Rahman D., Pappin D. J., Trowsdale J., Freemont P. S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler A. E., Matile H., Gassmann U., Engels M., Wyler R. European isolates of bovine herpesvirus 1: a comparison of restriction endonuclease sites, polypeptides, and reactivity with monoclonal antibodies. Arch Virol. 1985;85(1-2):57–69. doi: 10.1007/BF01317006. [DOI] [PubMed] [Google Scholar]
- Metzler A. E., Schudel A. A., Engels M. Bovine herpesvirus 1: molecular and antigenic characteristics of variant viruses isolated from calves with neurological disease. Arch Virol. 1986;87(3-4):205–217. doi: 10.1007/BF01315300. [DOI] [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Smith H. A., Straus S. E., Cohen J. I. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992 Dec;66(12):7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Ostrove J. M. Characterization of a potent varicella-zoster virus-encoded trans-repressor. J Virol. 1991 Oct;65(10):5289–5296. doi: 10.1128/jvi.65.10.5289-5296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson T., Everett R. D. Mutational dissection of the HSV-1 immediate-early protein Vmw175 involved in transcriptional transactivation and repression. Virology. 1988 Sep;166(1):186–196. doi: 10.1016/0042-6822(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Schwyzer M., Vlcek C., Menekse O., Fraefel C., Paces V. Promoter, spliced leader, and coding sequence for BICP4, the largest of the immediate-early proteins of bovine herpesvirus 1. Virology. 1993 Nov;197(1):349–357. doi: 10.1006/viro.1993.1596. [DOI] [PubMed] [Google Scholar]
- Stevenson D., Colman K. L., Davison A. J. Characterization of the varicella-zoster virus gene 61 protein. J Gen Virol. 1992 Mar;73(Pt 3):521–530. doi: 10.1099/0022-1317-73-3-521. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Wigdahl B. Identification of dominant-negative mutants of the herpes simplex virus type 1 immediate-early protein ICP0. J Virol. 1992 Apr;66(4):2261–2267. doi: 10.1128/jvi.66.4.2261-2267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth U. V., Fraefel C., Vogt B., Vlcek C., Paces V., Schwyzer M. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3' coterminal and encode a putative zinc finger transactivator protein. J Virol. 1992 May;66(5):2763–2772. doi: 10.1128/jvi.66.5.2763-2772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth U. V., Vogt B., Schwyzer M. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J Virol. 1991 Jan;65(1):195–205. doi: 10.1128/jvi.65.1.195-205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Heuchel R., Schaffner W., Kägi J. H. Thionein (apometallothionein) can modulate DNA binding and transcription activation by zinc finger containing factor Sp1. FEBS Lett. 1991 Feb 25;279(2):310–312. doi: 10.1016/0014-5793(91)80175-3. [DOI] [PubMed] [Google Scholar]