Abstract
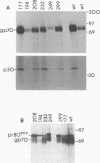
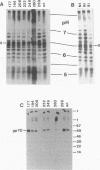
Virus-induced cell fusion of the fusion-from-without type was observed in SC-1 cells infected with Moloney murine leukemia virus when grown in NIH 3T3 cells. Replication-competent virus mutants with altered surface protein gp70 were examined. Fusion mutations were found in the proline-rich region of gp70. They acted on a step after binding and before or during endocytosis. The fusion mutants had an altered gp70 isomer pattern, presumably caused by different glycosylation. Other mutants with deleted glycans were analyzed, and some which also showed defective fusion were found. The interrelationship of the proline-rich region, glycosylation, and fusion is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Kim J. W., Tseng L., Cunningham J. M. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J Virol. 1993 Apr;67(4):2091–2096. doi: 10.1128/jvi.67.4.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K. B. Cleavage fragments of the retrovirus surface protein gp70 during virus entry. J Gen Virol. 1987 Aug;68(Pt 8):2193–2202. doi: 10.1099/0022-1317-68-8-2193. [DOI] [PubMed] [Google Scholar]
- Andersen K. B., Nexø B. A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983 Feb;125(1):85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Andersen K. B., Skov H. Retrovirus-induced cell fusion is enhanced by protease treatment. J Gen Virol. 1989 Jul;70(Pt 7):1921–1927. doi: 10.1099/0022-1317-70-7-1921. [DOI] [PubMed] [Google Scholar]
- Battini J. L., Heard J. M., Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992 Mar;66(3):1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J., Goff S. P. Mutants and pseudorevertants of Moloney murine leukemia virus with alterations at the integration site. Cell. 1985 Sep;42(2):573–580. doi: 10.1016/0092-8674(85)90114-x. [DOI] [PubMed] [Google Scholar]
- Dedera D. A., Gu R. L., Ratner L. Role of asparagine-linked glycosylation in human immunodeficiency virus type 1 transmembrane envelope function. Virology. 1992 Mar;187(1):377–382. doi: 10.1016/0042-6822(92)90331-i. [DOI] [PubMed] [Google Scholar]
- Felkner R. H., Roth M. J. Mutational analysis of the N-linked glycosylation sites of the SU envelope protein of Moloney murine leukemia virus. J Virol. 1992 Jul;66(7):4258–4264. doi: 10.1128/jvi.66.7.4258-4264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer J., Turnock G. Glycoproteins from murine C-type virus are more acidic in virus derived from transformed cells than from nontransformed cells. Virology. 1978 Jul 1;88(1):177–182. doi: 10.1016/0042-6822(78)90121-6. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J. Identification and characterization of fusion and processing domains of the human immunodeficiency virus type 2 envelope glycoprotein. J Virol. 1992 Sep;66(9):5472–5478. doi: 10.1128/jvi.66.9.5472-5478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Identification of the principal neutralizing determinant of human immunodeficiency virus type 1 as a fusion domain. J Virol. 1991 Jan;65(1):190–194. doi: 10.1128/jvi.65.1.190-194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Risser R. Identification of conserved residues in the human immunodeficiency virus type 1 principal neutralizing determinant that are involved in fusion. AIDS Res Hum Retroviruses. 1991 Oct;7(10):807–811. doi: 10.1089/aid.1991.7.807. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Analytical isoelectric focusing using a high-voltage vertical slab polyacrylamide gel system. Anal Biochem. 1984 Nov 1;142(2):421–436. doi: 10.1016/0003-2697(84)90486-x. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. S., Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993 Jan;67(1):67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Kayman S. C., Kopelman R., Projan S., Kinney D. M., Pinter A. Mutational analysis of N-linked glycosylation sites of Friend murine leukemia virus envelope protein. J Virol. 1991 Oct;65(10):5323–5332. doi: 10.1128/jvi.65.10.5323-5332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastromarino P., Conti C., Petruzziello C., Lapadula R., Orsi N. Effect of electric charged molecules on Sindbis virus hemagglutination and hemolysis. Microbiologica. 1992 Jan;15(1):23–28. [PubMed] [Google Scholar]
- McClure M. O., Sommerfelt M. A., Marsh M., Weiss R. A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990 Apr;71(Pt 4):767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- Nexo B. A. A plaque assay for murine leukemia virus using enzyme-coupled antibodies. Virology. 1977 Apr;77(2):849–852. doi: 10.1016/0042-6822(77)90504-9. [DOI] [PubMed] [Google Scholar]
- Nexø B. A., Ulrich K. Variants of type-C retroviruses from DBA/2 mice: protein-structural and biological properties. Virology. 1983 Mar;125(2):454–467. doi: 10.1016/0042-6822(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Page K. A., Stearns S. M., Littman D. R. Analysis of mutations in the V3 domain of gp160 that affect fusion and infectivity. J Virol. 1992 Jan;66(1):524–533. doi: 10.1128/jvi.66.1.524-533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti M., DeLeo A. B., Pinter A., O'Donnell P. V., Hämmerling U., Fleissner E. The GIX antigen of murine leukemia virus: an analysis with monoclonal antibodies. Virology. 1981 Jul 30;112(2):450–460. doi: 10.1016/0042-6822(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. O-linked glycosylation of retroviral envelope gene products. J Virol. 1988 Mar;62(3):1016–1021. doi: 10.1128/jvi.62.3.1016-1021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tung J. S., O'Donnell P. V., Hämmerling U. Structural domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology. 1982 Jan 30;116(2):499–516. doi: 10.1016/0042-6822(82)90143-x. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Tung J. S., Hopkins N., Robbins P. W. Relationship of GIX antigen expression to the glycosylation of murine leukemia virus glycoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6420–6424. doi: 10.1073/pnas.77.11.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Roy-Burman P., Dougherty M., Pal B. K., Charman H. P., Klement V., Gardner M. B. Assay for type C virus in mouse sera based on particulate reverse transcriptase activity. J Virol. 1976 Sep;19(3):1107–1110. doi: 10.1128/jvi.19.3.1107-1110.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMKOVIC D., SVOBODA J., VALENTOVA N. Clonal analysis of line XC-tc rat tumour cells (derived from tumour XC) grown in vitro. Folia Biol (Praha) 1963 Apr;9:82–91. [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Skov H., Andersen K. B. Mutational analysis of Moloney murine leukaemia virus surface protein gp70. J Gen Virol. 1993 Apr;74(Pt 4):707–714. doi: 10.1099/0022-1317-74-4-707. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- Wilson C. A., Marsh J. W., Eiden M. V. The requirements for viral entry differ from those for virally induced syncytium formation in NIH 3T3/DTras cells exposed to Moloney murine leukemia virus. J Virol. 1992 Dec;66(12):7262–7269. doi: 10.1128/jvi.66.12.7262-7269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., Kaufman S. J. Mechanism of murine leukemia virus-induced fusion in rat myoblasts defective in differentiation. J Virol. 1977 Sep;23(3):768–775. doi: 10.1128/jvi.23.3.768-775.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling D. A., Keshet I. Fusion activity of virions of murine leukemia virus. Virology. 1979 May;95(1):185–196. doi: 10.1016/0042-6822(79)90413-6. [DOI] [PubMed] [Google Scholar]