Abstract
Recent paleontological discoveries in Madagascar document the existence of a diverse clade of palaeopropithecids or “sloth lemurs”: Mesopropithecus (three species), Babakotia (one species), Palaeopropithecus (three species), and Archaeoindris (one species). This mini-radiation of now extinct (“subfossil”) lemurs is most closely related to the living indrids (Indri, Propithecus, and Avahi). Whereas the extant indrids are known for their leaping acrobatics, the palaeopropithecids (except perhaps for the poorly known giant Archaeoindris) exhibit numerous skeletal design features for antipronograde or suspensory positional behaviors (e.g., high intermembral indices and mobile joints). Here we analyze the curvature of the proximal phalanges of the hands and feet. Computed as the included angle (θ), phalangeal curvature develops in response to mechanical use and is known to be correlated in primates with hand and foot function in different habitats; terrestrial species have straighter phalanges than their arboreal counterparts, and highly suspensory forms such as the orangutan possess the most curved phalanges. Sloth lemurs as a group are characterized by very curved proximal phalanges, exceeding those seen in spider monkeys and siamangs, and approaching that of orangutans. Indrids have curvatures roughly half that of sloth lemurs, and the more terrestrial, subfossil Archaeolemur possesses the least curved phalanges of all the indroids. Taken together with many other derived aspects of their postcranial anatomy, phalangeal curvature indicates that the sloth lemurs are one of the most suspensory clades of mammals ever to evolve.
Keywords: primate evolution, locomotion, bone curvature
Lesser apes and orangutans in Asia and spider monkeys in South America are the most suspensory, arboreal primates alive today. In the fossil record of higher primates, perhaps only the Miocene ape Oreopithecus bambolii from Italy exhibits comparably antipronograde adaptations (1–3). The isolated island of Madagascar also produced its own family of highly suspensory primates that persisted late into the Holocene—the palaeopropithecids or “sloth lemurs” (4–8).
The most extreme expression of this suspensory design is seen in the postcranial skeleton of Palaeopropithecus (9–14). The femur of this “subfossil” genus is so derived, relative to its living indrid relatives, that the first one discovered was attributed initially to “Bradytherium” by Grandidier (15), who believed it was from a previously unknown Malagasy sloth. There was much ensuing taxonomic confusion that was resolved in large part by Carleton ( ref. 9, “convergent evolution” was favored over some inexplicable biogeographical scenario) and completely by Lamberton (ref. 16; e.g., many dental similarities link Palaeopropithecus closely to living indrids). Only recently, however, with the addition of newly collected specimens from the cave deposits of northern Madagascar, did it become apparent that there was an entire radiation of sloth-like lemurs: Palaeopropithecus (two recognized species, probably a third), Babakotia (one species), Mesopropithecus (three recognized species), and Archaeoindris (one species). Postcranial proportions and anatomy indicate that the first three genera were primarily arboreal and suspensory to varying degrees (4, 5, 8, 9, 14, 16–18). Regrettably little is known about the locomotor skeleton of the giant Archaeoindris (19, 20), but its large body size (≈200 kg) and femoral anatomy have evoked analogies to ground sloths (13, 21).
Phalangeal Curvature and Primate Locomotor Adaptations
Despite the many postcranial convergences between sloths and palaeopropithecids, this functional analogy cannot be extended without qualification to their hands and feet. Sloths possess long, curved claws on their digits whereas sloth lemurs, like most primates, bear short, flattened nails on their distal phalanges. Both groups sport analogous, hook-like hands and feet, but the comparative set for sloth lemurs is best limited to other primates [although variation in claw curvature is also correlated with habitat differences (22)]. The recovery of new and abundant elements of sloth lemur hands and feet permits us to re-examine questions of substrate use and positional behavior in this group. The hands and feet contact the structural environment directly and form the biomechanical link through which forces are transferred between the animal and the physical environment. As such, their design should faithfully reflect habitual stresses and the adaptive responses to these mechanical stimuli (23–25). Perhaps nowhere is this structure–function connection more apparent in other primates than in the shape of the proximal phalanges of the hands and feet.
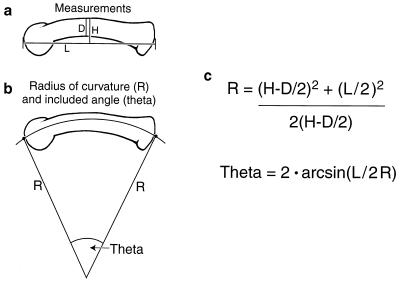
Biomechanical theory and experimental evidence indicate that bone curvature is closely and necessarily linked to functional demands, including postnatal stresses (26) and prenatal stimuli (27, 28). Curved phalanges of primates are assumed by most to be associated with their obvious gripping function in an arboreal habitat (e.g., refs. 29 and 30; see ref. 31 for a review). The predictability of this relationship has permitted primate paleontologists to reconstruct habitats and locomotor adaptations in fossil species (e.g., refs. 32–37). Efforts of this nature have benefited from the ability to quantify phalangeal curvature easily and reliably (31). For example, the included angle (θ in Fig. 1) is calculated from only three measurements: interarticular length (L), dorsovolar midshaft diameter (D), and projected height (H). Terrestrial anthropoid species (both quadrupeds and bipeds) have relatively straight phalanges (low values of θ), and highly suspensory, arboreal species possess very curved bones (high values of θ); species that utilize both arboreal and terrestrial substrates tend to be characterized by intermediate curvatures (31, 35).
Figure 1.
(a) Measurements needed to calculate (b) radius of curvature R and included angle of curvature θ; (c) calculations of R and θ.
Are ecomorphological inferences based on the hands and feet of sloth lemurs congruent with those derived from body shape, long bone geometry, and axial skeletal anatomy that favor the reconstruction of a highly suspensory behavioral repertoire? How did Babakotia, Mesopropithecus, and Palaeopropithecus compare in this regard? And how do the palaeopropithecids compare with their extant relatives and to the most suspensory living anthropoid primates?
Materials and Methods
We have calculated phalangeal curvature (as θ) for sloth lemurs using this method, and place our results into a broadly comparative context by contrasting them with a variety of living anthropoid and prosimian primates of known positional repertoires. The Palaeopropithecus sample includes 69 proximal phalanges divided into 3 groups: 13 from Palaeopropithecus maximus (central plateau); 45 from Palaeopropithecus ingens (southern and southwest); and 11 phalanges of an undescribed new species from Anjohibe Cave near Mahajanga in the northwest, 10 of which are associated phalanges from the most complete specimen ever recovered (38). The sample of Babakotia radofilai consists of 38 proximal phalanges, 12 of which are from an associated individual (DPC-10994), all from the caves of the Ankarana Massif (5). Only 5 proximal phalanges comprise the Mesopropithecus sample, and most are recent finds from a single individual of a new species, Mesopropithecus dolichobrachion (18).
Although it was possible to sort hand from foot phalanges in Babakotia with some confidence based on basal articular shape, it proved difficult to sort reliably isolated phalanges of Palaeopropithecus. However, curvatures of the manual and pedal phalanges of Babakotia and many of the living species (e.g., indrids) considered here are not significantly different; therefore, phalanges were pooled in all subfossil and extant comparisons to follow. The extant comparative example includes the largest living indroid relatives of the sloth lemurs, Indri indri and Propithecus diadema, as well as the largest extant lemurid, Varecia variegata. Several highly suspensory anthropoid species are also included (and which more closely approximate the estimated body masses of palaeopropithecids, ref. 8): the orangutan, Pongo pygmaeus; the siamang, Hylobates syndactylus (hand only); and the spider monkey, Ateles (mixed species). Two species of the arboreal–terrestrial African apes are also included (Pan paniscus and Pan troglodytes), along with the largest colobine (Nasalis larvatus), and pedal phalanges of the primarily terrestrial baboons (Papio hamadryas). Finally, phalanges from another subfossil lemur, Archaeolemur, are included in the comparisons; this species is believed to have been the most terrestrial, “monkey-like” of the subfossil indroids (8). The total number of phalanges measured is 808, of which 140 are from extinct lemurs.
Our results are presented for the indroids in tabular form, including sample size, mean, standard error of the mean, and coefficient of variation. ANOVA was used with raw and ranked data to test the null hypotheses that degree of curvature does not differ significantly within indroids and within palaeopropithecids. Comparisons are then extended graphically to include the other extant species listed above. The mean is indicated by a circle or star (the latter identifies species of sloth lemurs), and ±1 SD are plotted to provide a sense of dispersion around the central tendencies.
Phalangeal Curvature and Positional Behavior in Sloth Lemurs
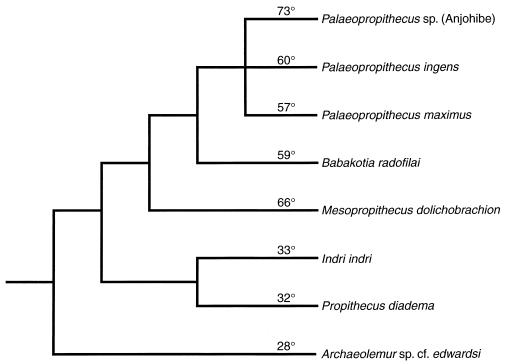
There is statistically significant variation in the degree of phalangeal curvature present in the indroid sample (P < 0.001). The data summarized in Table 1 indicate that Archaeolemur possesses the least curved phalanges and the Anjohibe Palaeopropithecus has the most curved; all of the sloth lemurs have significantly more curved bones than either Archaeolemur or the living indrids (see also Fig. 2). These data are mapped onto a cladogram of indroid relationships in Fig. 3 (4), and it is clear that a very high degree of curvature is a shared-derived character linking all palaeopropithecids. ANOVA also reveals significant heterogeneity in curvature within the palaeopropithecids (P < 0.01), but post hoc multiple comparisons indicate that it is only the Anjohibe Palaeopropithecus sample that is different from (i.e., more curved than) other sloth lemurs, including P. ingens and P. maximus. Best known for their leaping prowess, Indri and P. diadema also practice a variety of suspensory behaviors, yet their curvature is only roughly half that of the sloth lemurs. As the most terrestrial indroid considered here, Archaeolemur’s least degree of curvature is consistent with other postcranial evidence that suggests pronograde positional behavior was the dominant element in this animal’s repertoire (8).
Table 1.
Phalangeal curvature (θ) in indroid primates
| Species | Phalanges, n | Mean θ, degrees | SEM | Coefficient of variation |
|---|---|---|---|---|
| I. indri | 130 | 33.1 | 0.54 | 18.7 |
| P. diadema | 51 | 31.8 | 0.74 | 16.7 |
| M. dolichobrachion | 5 | 65.8 | 2.99 | 10.2 |
| B. radofilai | 38 | 59.1 | 1.02 | 10.7 |
| P. ingens | 45 | 60.3 | 1.48 | 16.4 |
| P. maximus | 13 | 57.2 | 2.72 | 17.1 |
| Palaeopropithecus sp. (Anjohibe) | 11 | 73.3 | 2.20 | 10.0 |
| Archaeolemur sp. cf. edwardsi | 28 | 27.9 | 1.19 | 22.6 |
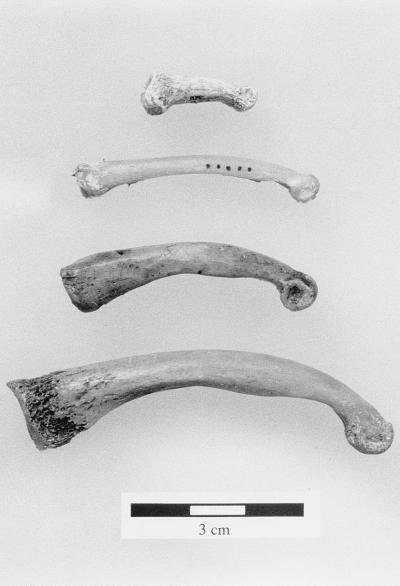
Figure 2.
Representative examples of proximal phalanges of indroid primates (top to bottom: Archaeolemur, Indri, Babakotia, and Palaeopropithecus).
Figure 3.
Cladogram of indroid primates with average degree of phalangeal curvature indicated. A high degree of phalangeal curvature is a synapomorphy of the sloth lemurs (palaeopropithecids).
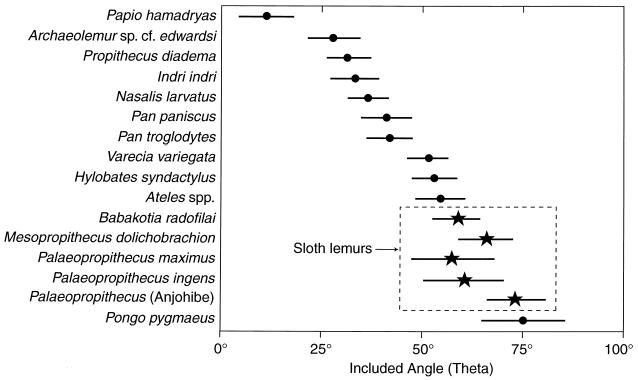
In the expanded comparisons presented in Fig. 4, it is apparent that all living, highly suspensory primates tend to possess very curved bones in comparison to less suspensory, more pronograde, and terrestrial species. Quadrumanous orangutans and the highly terrestrial baboons exhibit the maximum differences observed in this extant sample. Arboreality and antipronogrady result in very curved phalanges regardless of phylogenetic affinities (e.g., compare the Neotropical spider monkey to the siamang). Both pygmy and common chimpanzees are versatile arborealists that engage in suspensory locomotion and feeding postures but usually travel on the ground (39, 40), and they have phalanges less curved than orangutans but more curved than Nasalis, a nonsuspensory arboreal quadruped. Varecia is now known to also be quite suspensory (41, 42) and possesses phalanges much more curved on average than those of the two living indrids. Suspensory behaviors probably characterized the last common ancestor of indrids and palaeopropithecids (4) and may have been primitive for lemurids and indroids (14). If so, then Varecia may approximate the primitive condition more closely than do the leaping indrids (including the degree of phalangeal curvature).
Figure 4.
Curvature of the proximal phalanges in living primates and subfossil indroids. The average for θ is indicated (★ for sloth lemurs, • for all other species) along with ±1 SD. Note the high degree of curvature in all species of extinct sloth lemurs.
Average degree of curvature appears inversely related to body size in Palaeopropithecus (the Anjohibe species has the smallest body size but greatest curvature, P. maximus has the largest body size and the least curvature). The Anjohibe species is the only primate to approximate the extreme degree of curvature seen in the orangutan, and curvature in the other members of this genus exceeds all other primates. Other suspensory adaptations in the skeleton of Palaeopropithecus are well known and corroborate the inferences drawn here from the degree of phalangeal curvature (8–11, 14). Other lines of evidence indicate that M. dolichobrachion is probably the most suspensory species of Mesopropithecus (18), and its phalangeal curvature also exceeds that of all extant primates except for the orangutan. The highly curved phalanges of Babakotia are also consistent with a suite of other postcranial features linked to suspensory behaviors (e.g., high intermembral index, large spherical femoral head lacking a fovea capitis, reduced malleoli, reduced hindfoot, etc.). In sum, all of the sloth lemurs exhibit an extreme degree of phalangeal curvature compared with other primates, including highly suspensory forms such as the spider monkey, siamang, and chimpanzees. Although many other parts of the skeletons of sloth lemurs find their closest analogues in true sloths, it is the large-bodied, highly suspensory orangutan that compares most favorably to this group in the shape of the phalanges (see refs. 10 and 11). As a group, it appears that the sloth lemurs are among the most suspensory clades of mammals ever to evolve.
Acknowledgments
We thank the government of Madagascar and the University of Antananario for its sponsorship under which the Duke University Primate Center and Stony Brook protocols of collaboration operate. Special thanks go to Dr. Berthe Rakotosamimanana and Benjamin Andriamihaja for their continuing support and cooperation in Madagascar. Access to collections was graciously provided by Peter Andrews and Paula Jenkins (London), Renate Angermann (Berlin), Ross MacPhee (New York), Chris Smeenk (Leiden), Maria Rutzmoser (Cambridge), and Bruce Latimer (Cleveland). We also thank Jack Stern and Randall Susman for sharing their data on apes. The research reported here was supported by National Science Foundation Grants BNS-8911315 and SBR-9630350, the Boise Fund, and the School of Medicine at Stony Brook. This is Duke University Primate Center publication no. 649.
References
- 1.Simons E L. Primate Evolution: An Introduction to Man’s Place Nature. New York: MacMillan; 1972. [Google Scholar]
- 2.Jungers W L. J Hum Evol. 1987;16:445–456. [Google Scholar]
- 3.Sarmiento E E. Am Mus Novitat. 1987;2881:1–44. [Google Scholar]
- 4.Jungers W L, Godfrey L R, Simons E L, Chatrath P S, Rakotosamimanana B. Proc Natl Acad Sci USA. 1991;88:9082–9086. doi: 10.1073/pnas.88.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons E L, Godfrey L R, Jungers W L, Chatrath P S, Rakotosamimanana B. Folia Primatol. 1992;58:197–203. [Google Scholar]
- 6.Simons E L, Burney D A, Chatrath P S, Godfrey L R, Jungers W L. Quat Res. 1995;43:249–254. [Google Scholar]
- 7.Tattersall I. The Primates of Madagascar. New York: Columbia Univ. Press; 1982. [Google Scholar]
- 8.Godfrey L R, Jungers W L, Reed K E, Simons E L, Chatrath P S. In: Natural Change and Human Impact in Madagascar. Goodman S M, Patterson B D, editors. Washington, DC: Smithsonian Institution Press; 1997. pp. 218–256. [Google Scholar]
- 9.Carleton A. Proc Zool Soc London. 1936;106:281–307. [Google Scholar]
- 10.Walker, A. C. (1967) Doctoral thesis (Univ. College, London).
- 11.Walker A C. In: Primate Locomotion. Jenkins F A, editor. New York: Academic; 1974. pp. 39–381. [Google Scholar]
- 12.Ekblom T. Bull Geol Instit Univ Uppsala. 1951;34:123–190. [Google Scholar]
- 13.Jungers W L. Z Morphol Anthrop. 1980;71:177–186. [PubMed] [Google Scholar]
- 14.Godfrey L R. J Hum Evol. 1988;17:93–134. [Google Scholar]
- 15.Grandidier G. Bull Mus Nat Hist Nat (Paris) 1902;8:497–502. [Google Scholar]
- 16.Lamberton C. Bull Acad Malgache. 1944-45;26:89–140. [Google Scholar]
- 17.Hamrick, M. W. (1995) Doctoral thesis. (Northwestern Univ., Chicago).
- 18.Simons E L, Godfrey L R, Jungers W L, Chatrath P S, Ravaoarisoa J. Int J Primatol. 1995;16:653–682. [Google Scholar]
- 19.Lamberton C. Mem Acad Malgache. 1934;17:1–39. [Google Scholar]
- 20.Vuillaume-Randriamanantena M. J Hum Evol. 1988;17:379–391. [Google Scholar]
- 21.Lamberton C. Bull Acad Malgache. 1946;27:24–28. [Google Scholar]
- 22.Feduccia A. Science. 1993;259:790–793. doi: 10.1126/science.259.5096.790. [DOI] [PubMed] [Google Scholar]
- 23.Jouffroy F K, Lessertisseur J. In: Environment, Behavior, and Morphology: Dynamic Interactions in Primates. Morbeck M E, Preuschoft H, Gomberg N, editors. New York: Fischer; 1979. pp. 143–181. [Google Scholar]
- 24.Lewis O J. Functional Morphology of the Evolving Hand and Foot. Oxford: Clarendon; 1989. [Google Scholar]
- 25.Gebo D L. In: Postcranial Adaptation in Nonhuman Primates. Gebo D L, editor. DeKalb: Northern Illinois Press; 1993. pp. 220–234. [Google Scholar]
- 26.Lanyon L E. J Zool London. 1980;192:457–466. [Google Scholar]
- 27.Hall B K, Herring S W. J Morphol. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- 28.Herring S W. In: Bone. Hall B K, editor. Vol. 9. Boca Raton, FL: CRC; 1994. pp. 165–191. [Google Scholar]
- 29.Oxnard C E. Form and Pattern in Human Evolution. Chicago: Univ. of Chicago Press; 1973. [Google Scholar]
- 30.Stern J T, Jr, Susman R L. Am J Phys Anthropol. 1983;60:279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- 31.Stern J T, Jr, Jungers W L, Susman R L. Am J Phys Anthropol. 1995;97:1–10. doi: 10.1002/ajpa.1330970102. [DOI] [PubMed] [Google Scholar]
- 32.Napier J R, Davis P R. Fossil Mammals of Africa. London: Br. Mus. Nat. Hist.; 1959. , No. 16. [Google Scholar]
- 33.Preuschoft H. Proceedings of the 3rd International Congress of Primatology, Zurich 1970. Vol. 1. Basel: Karger; 1971. pp. 79–90. [Google Scholar]
- 34.Preuschoft H. Proceedings of the 5th Congress of the International Primatology Society, Nagoya 1974. Vol. 3. New York: Academic; 1975. pp. 345–359. [Google Scholar]
- 35.Susman R L, Stern J T, Jr, Jungers W L. Folia Primatol. 1984;43:113–156. doi: 10.1159/000156176. [DOI] [PubMed] [Google Scholar]
- 36.Godinot M, Beard K C. Hum Evol. 1991;6:307–354. [Google Scholar]
- 37.Hamrick M W, Meldrum D J, Simons E L. J Hum Evol. 1995;28:121–145. [Google Scholar]
- 38.MacPhee R D E, Simons E L, Wells N A, Vuillaume-Randriamanantena M. Geotimes. Jan.; 1984. 10–11. [Google Scholar]
- 39.Hunt K D. Am J Phys Anthropol. 1991;86:521–536. doi: 10.1002/ajpa.1330860408. [DOI] [PubMed] [Google Scholar]
- 40.Doran D. Am J Phys Anthropol. 1993;91:83–98. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- 41.Dagosto M. Am J Phys Anthropol. 1994;94:189–202. doi: 10.1002/ajpa.1330940204. [DOI] [PubMed] [Google Scholar]
- 42.Meldrum D J, Dagosto M, White J. Am J Phys Anthropol. 1997;103:85–102. doi: 10.1002/(SICI)1096-8644(199705)103:1<85::AID-AJPA6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]