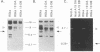Abstract
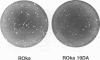
Cells infected with varicella-zoster virus (VZV) express a viral ribonucleotide reductase which is distinct from that present in uninfected cells. VZV open reading frames 18 and 19 (ORF18 and ORF19) are homologous to the herpes simplex virus type 1 genes encoding the small and large subunits of ribonucleotide reductase, respectively. We generated recombinant VZV by transfecting cultured cells with four overlapping cosmid DNAs. To construct a virus lacking ribonucleotide reductase, we deleted 97% of VZV ORF19 from one of the cosmids. Transfection of this cosmid with the other parental cosmids yielded a VZV mutant with a 2.3-kbp deletion confirmed by Southern blot analysis. Virus-specific ribonucleotide reductase activity was not detected in cells infected with VZV lacking ORF19. Infection of melanoma cells with ORF19-deleted VZV resulted in plaques smaller than those produced by infection with the parental VZV. The mutant virus also exhibited a growth rate slightly slower than that of the parental virus. Chemical inhibition of the VZV ribonucleotide reductase has been shown to potentiate the anti-VZV activity of acyclovir. Similarly, the concentration of acyclovir required to inhibit plaque formation by 50% was threefold lower for the VZV ribonucleotide reductase deletion mutants than for parental virus. We conclude that the VZV ribonucleotide reductase large subunit is not essential for virus infection in vitro; however, deletion of the gene impairs the growth of VZV in cell culture and renders the virus more susceptible to inhibition by acyclovir.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron J. M., McDougall I., Marsden H. S., Preston V. G., Ryan D. M., Subak-Sharpe J. H. Ribonucleotide reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J Gen Virol. 1988 Oct;69(Pt 10):2607–2612. doi: 10.1099/0022-1317-69-10-2607. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Goldstein D. J., Weller S. K. Herpes simplex virus ribonucleotide reductase mutants are hypersensitive to acyclovir. Antimicrob Agents Chemother. 1989 Aug;33(8):1395–1399. doi: 10.1128/aac.33.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Seidel K. E. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7376–7380. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C. V., Costa J. V. Induction of ribonucleotide reductase activity in cells infected with African swine fever virus. Virology. 1992 Mar;187(1):73–83. doi: 10.1016/0042-6822(92)90296-2. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Varicella-zoster virus. The Fourteenth Fleming lecture. J Gen Virol. 1991 Mar;72(Pt 3):475–486. doi: 10.1099/0022-1317-72-3-475. [DOI] [PubMed] [Google Scholar]
- Dutia B. M. Ribonucleotide reductase induced by herpes simplex virus has a virus-specified constituent. J Gen Virol. 1983 Mar;64(Pt 3):513–521. doi: 10.1099/0022-1317-64-3-513. [DOI] [PubMed] [Google Scholar]
- Engström Y., Rozell B., Hansson H. A., Stemme S., Thelander L. Localization of ribonucleotide reductase in mammalian cells. EMBO J. 1984 Apr;3(4):863–867. doi: 10.1002/j.1460-2075.1984.tb01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrin L. J., Camerini-Otero R. D. Selective cleavage of human DNA: RecA-assisted restriction endonuclease (RARE) cleavage. Science. 1991 Dec 6;254(5037):1494–1497. doi: 10.1126/science.1962209. [DOI] [PubMed] [Google Scholar]
- Gershon A. A., LaRussa P., Hardy I., Steinberg S., Silverstein S. Varicella vaccine: the American experience. J Infect Dis. 1992 Aug;166 (Suppl 1):S63–S68. doi: 10.1093/infdis/166.supplement_1.s63. [DOI] [PubMed] [Google Scholar]
- Gibson T., Stockwell P., Ginsburg M., Barrell B. Homology between two EBV early genes and HSV ribonucleotide reductase and 38K genes. Nucleic Acids Res. 1984 Jun 25;12(12):5087–5099. doi: 10.1093/nar/12.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988 Sep;166(1):41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988 Jan;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Brunel P. A. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32 degrees C. Infect Immun. 1978 Jan;19(1):199–203. doi: 10.1128/iai.19.1.199-203.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy I., Gershon A. A., Steinberg S. P., LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med. 1991 Nov 28;325(22):1545–1550. doi: 10.1056/NEJM199111283252204. [DOI] [PubMed] [Google Scholar]
- Henry B. E., Glaser R., Hewetson J., O'Callaghan D. J. Expression of altered ribonucleotide reductase activity associated with the replication of the Epstein-Barr virus. Virology. 1978 Aug;89(1):262–271. doi: 10.1016/0042-6822(78)90058-2. [DOI] [PubMed] [Google Scholar]
- Huszar D., Bacchetti S. Partial purification and characterization of the ribonucleotide reductase induced by herpes simplex virus infection of mammalian cells. J Virol. 1981 Feb;37(2):580–588. doi: 10.1128/jvi.37.2.580-588.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J. G., Leib D. A., Goldstein D. J., Bogard C. L., Schaffer P. A., Weller S. K., Coen D. M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989 Nov;173(1):276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Lankinen H., Gräslund A., Thelander L. Induction of a new ribonucleotide reductase after infection of mouse L cells with pseudorabies virus. J Virol. 1982 Mar;41(3):893–900. doi: 10.1128/jvi.41.3.893-900.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R. S., Keller P. M., Keech B. J., Davison A. J., Whang Y., Morgan A. J., Kieff E., Ellis R. W. Varicella-zoster virus as a live vector for the expression of foreign genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3896–3900. doi: 10.1073/pnas.84.11.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Smith H. A., Straus S. E., Cohen J. I. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992 Dec;66(12):7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera L. P., Mosca J. D., Ruyechan W. T., Hay J. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J Virol. 1992 Sep;66(9):5298–5304. doi: 10.1128/jvi.66.9.5298-5304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon M., Eisenberg R. J., Cohen G. H. Ribonucleotide reductase from herpes simplex virus (types 1 and 2) infected and uninfected KB cells: properties of the partially purified enzymes. J Gen Virol. 1977 Jul;36(1):163–173. doi: 10.1099/0022-1317-36-1-163. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Darling A. J., McDougall I. M. The herpes simplex virus type 1 temperature-sensitive mutant ts1222 has a single base pair deletion in the small subunit of ribonucleotide reductase. Virology. 1988 Dec;167(2):458–467. [PubMed] [Google Scholar]
- Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993 Jun 18;260(5115):1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Hayakawa Y., Mori H., Namazue J., Takamizawa A., Yoshida I., Yamanishi K., Takahashi M. Development of immunogenic recombinant Oka varicella vaccine expressing hepatitis B virus surface antigen. J Gen Virol. 1991 Jun;72(Pt 6):1393–1399. doi: 10.1099/0022-1317-72-6-1393. [DOI] [PubMed] [Google Scholar]
- Slabaugh M. B., Johnson T. L., Mathews C. K. Vaccinia virus induces ribonucleotide reductase in primate cells. J Virol. 1984 Nov;52(2):507–514. doi: 10.1128/jvi.52.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Averett D. R., Nelson D. J., Lambe C. U., Morrison R. W., Jr, St Clair M. H., Furman P. A. Potentiation of antiherpetic activity of acyclovir by ribonucleotide reductase inhibition. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4254–4257. doi: 10.1073/pnas.82.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Harrington J. A., Morrison R. W., Jr, Lambe C. U., Nelson D. J., Averett D. R., Biron K., Furman P. A. 2-Acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (A1110U), a potent inactivator of ribonucleotide reductases of herpes simplex and varicella-zoster viruses and a potentiator of acyclovir. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1051–1055. doi: 10.1073/pnas.86.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Lobe D. C., Ellis M. N., Blumenkopf T. A., Szczech G. M. Inactivators of herpes simplex virus ribonucleotide reductase: hematological profiles and in vivo potentiation of the antiviral activity of acyclovir. Antimicrob Agents Chemother. 1992 May;36(5):934–937. doi: 10.1128/aac.36.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Stonehuerner J. G., Biron K. K., Averett D. R. Ribonucleotide reductase induced by varicella zoster virus. Characterization, and potentiation of acyclovir by its inhibition. Biochem Pharmacol. 1987 Dec 15;36(24):4341–4346. doi: 10.1016/0006-2952(87)90682-4. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Okuno Y., Otsuka T., Osame J., Takamizawa A. Development of a live attenuated varicella vaccine. Biken J. 1975 Mar;18(1):25–33. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Kimura H., Morishima T., Daikoku T., Maeno K., Nishiyama Y. The pathogenicity of ribonucleotide reductase-null mutants of herpes simplex virus type 1 in mice. J Infect Dis. 1991 Dec;164(6):1091–1097. doi: 10.1093/infdis/164.6.1091. [DOI] [PubMed] [Google Scholar]
- de Wind N., Berns A., Gielkens A., Kimman T. Ribonucleotide reductase-deficient mutants of pseudorabies virus are avirulent for pigs and induce partial protective immunity. J Gen Virol. 1993 Mar;74(Pt 3):351–359. doi: 10.1099/0022-1317-74-3-351. [DOI] [PubMed] [Google Scholar]