Abstract
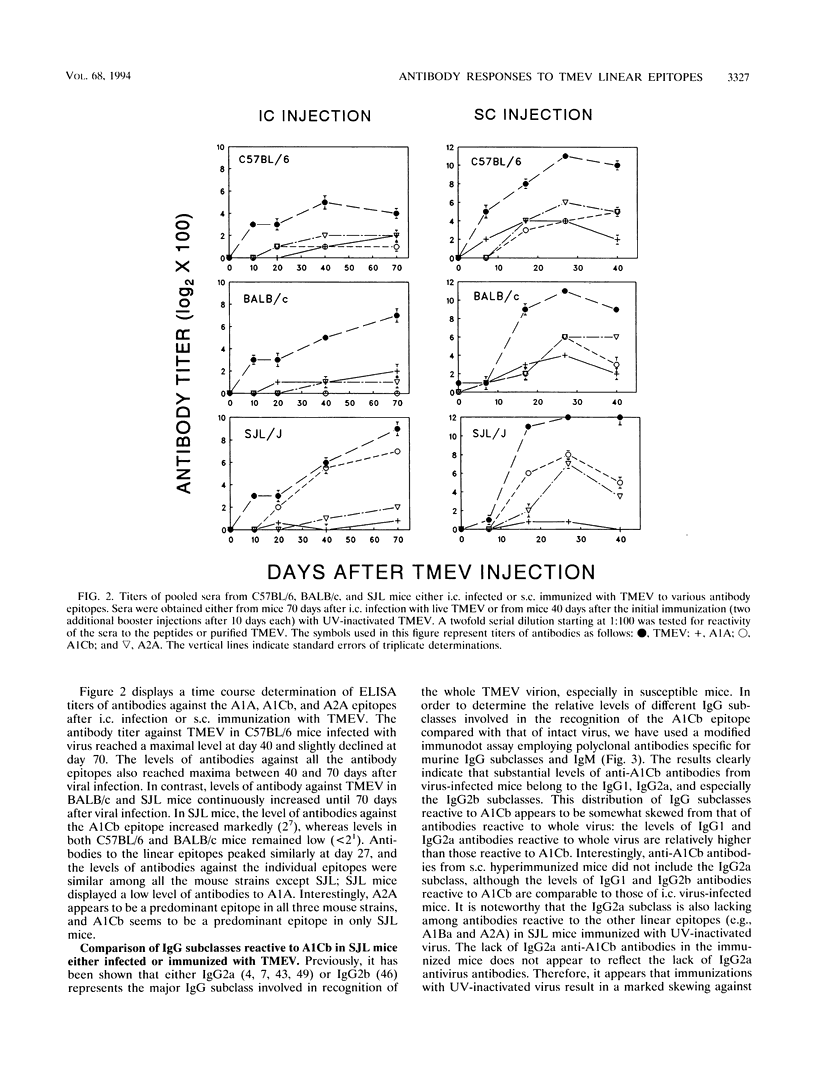
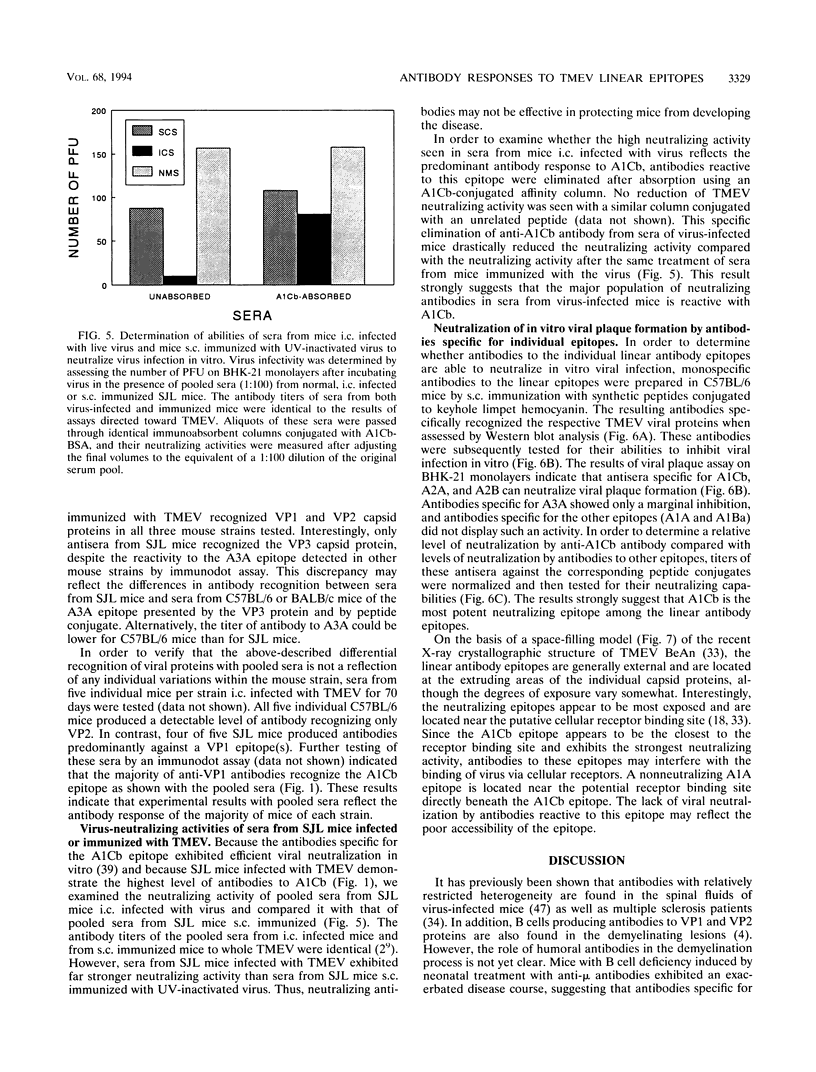
Using synthetic peptides, we have defined the major linear antibody epitopes of Theiler's murine encephalomyelitis virus (TMEV), i.e., A1A (VP1(12-25)), A1Ba (VP1(146-160)), A1Cb (VP1(262-276)), A2A (VP2(2-16)), A2B (VP2(165-179)), and A3A (VP3(24-37)). A time course study with either pooled or individual sera indicates that susceptible SJL mice intracerebrally infected with TMEV strongly and selectively recognize the A1Cb epitope of VP1, compared with resistant BALB/c or C57BL/6 mice, which broadly recognize most of the epitopes on the different capsid proteins. However, antibodies from SJL mice subcutaneously immunized with TMEV recognize primarily A1Ba, A1Cb, and A2A epitopes. A similar predominant recognition of the A1Cb epitope was found with antibodies from the cerebrospinal fluid of intracerebrally virus-infected SJL mice. Interestingly, a substantial level of antibodies against the A1Cb epitope in virus-infected SJL mice is of the immunoglobulin G2a subclass, in contrast to an undetectable level of this immunoglobulin G subclass in virus-immunized SJL mice. The level of in vitro viral neutralization by antibodies did not correlate with the clinical signs. Antibodies to A1Cb, A2A, and A2B were able to neutralize viral plaque formation in vitro, while antibodies to A3A, A1A, and A1Ba were not.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall S. S., Concannon P., Charmley P., McFarland H. F., Gatti R. A., Hood L. E., McFarlin D. E., Biddison W. E. The germline repertoire of T cell receptor beta-chain genes in patients with chronic progressive multiple sclerosis. J Neuroimmunol. 1989 Jan;21(1):59–66. doi: 10.1016/0165-5728(89)90159-8. [DOI] [PubMed] [Google Scholar]
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Cash E., Bandeira A., Chirinian S., Brahic M. Characterization of B lymphocytes present in the demyelinating lesions induced by Theiler's virus. J Immunol. 1989 Aug 1;143(3):984–988. [PubMed] [Google Scholar]
- Chow M., Yabrov R., Bittle J., Hogle J., Baltimore D. Synthetic peptides from four separate regions of the poliovirus type 1 capsid protein VP1 induce neutralizing antibodies. Proc Natl Acad Sci U S A. 1985 Feb;82(3):910–914. doi: 10.1073/pnas.82.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatch R. J., Lipton H. L., Miller S. D. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986 Feb 1;136(3):920–927. [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987 Jan 1;165(1):64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane M. A., Jue C., Mitchell M., Lipton H., Kim B. S. Detection of restricted predominant epitopes of Theiler's murine encephalomyelitis virus capsid proteins expressed in the lambda gt11 system: differential patterns of antibody reactivity among different mouse strains. J Neuroimmunol. 1990 May;27(2-3):173–186. doi: 10.1016/0165-5728(90)90067-w. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Fleming J. O., Ting J. Y., Stohlman S. A., Weiner L. P. Improvements in obtaining and characterizing mouse cerebrospinal fluid. Application to mouse hepatitis virus-induced encephalomyelitis. J Neuroimmunol. 1983 Apr;4(2):129–140. doi: 10.1016/0165-5728(83)90017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann A., Lorch Y. Theiler's virus infection: a model for multiple sclerosis. Prog Med Virol. 1985;31:43–83. [PubMed] [Google Scholar]
- Giavedoni L. D., Kaplan G., Marcovecchio F., Piccone M. E., Palma E. L. Protection conferred by TrpE fusion proteins containing portions of the C-terminal region of capsid protein VP1 of foot-and-mouth disease virus. J Gen Virol. 1991 Apr;72(Pt 4):967–971. doi: 10.1099/0022-1317-72-4-967. [DOI] [PubMed] [Google Scholar]
- Grant R. A., Filman D. J., Fujinami R. S., Icenogle J. P., Hogle J. M. Three-dimensional structure of Theiler virus. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2061–2065. doi: 10.1073/pnas.89.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoatlin M. E., Kew O. M., Renz M. E. Regions of poliovirus protein VP1 produced in Escherichia coli induce neutralizing antibodies. J Virol. 1987 May;61(5):1442–1447. doi: 10.1128/jvi.61.5.1442-1447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. H., Mitchell M., Hamparian V. V. Rhinoviruses: kinetics of ultraviolet inactivation and effects of UV and heat on immunogenicity. Arch Virol. 1979;61(4):313–319. doi: 10.1007/BF01315018. [DOI] [PubMed] [Google Scholar]
- Kappel C. A., Dal Canto M. C., Melvold R. W., Kim B. S. Hierarchy of effects of the MHC and T cell receptor beta-chain genes in susceptibility to Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Immunol. 1991 Dec 15;147(12):4322–4326. [PubMed] [Google Scholar]
- Kappel C. A., Melvold R. W., Kim B. S. Influence of sex on susceptibility in the Theiler's murine encephalomyelitis virus model for multiple sclerosis. J Neuroimmunol. 1990 Sep-Oct;29(1-3):15–19. doi: 10.1016/0165-5728(90)90143-b. [DOI] [PubMed] [Google Scholar]
- Kim B. S., Choe Y. K., Crane M. A., Jue C. R. Identification and localization of a limited number of predominant conformation-independent antibody epitopes of Theiler's murine encephalomyelitus virus. Immunol Lett. 1992 Feb;31(2):199–205. doi: 10.1016/0165-2478(92)90146-f. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Pepys M. B., Kitajima K., Askonas B. A. Activation of mouse complement by different classes of mouse antibody. Immunology. 1979 Dec;38(4):687–695. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrich J. R., Arnason B. G., Hochberg F. H. Demyelinative myelopathy in mice induced by the DA virus. J Neurol Sci. 1976 Oct;29(2-4):149–160. doi: 10.1016/0022-510x(76)90167-2. [DOI] [PubMed] [Google Scholar]
- Levine B., Hardwick J. M., Trapp B. D., Crawford T. O., Bollinger R. C., Griffin D. E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991 Nov 8;254(5033):856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Calenoff M., Bandyopadhyay P., Miller S. D., Dal Canto M. C., Gerety S., Jensen K. The 5' noncoding sequences from a less virulent Theiler's virus dramatically attenuate GDVII neurovirulence. J Virol. 1991 Aug;65(8):4370–4377. doi: 10.1128/jvi.65.8.4370-4377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Chronic neurologic disease in Theiler's virus infection of SJL/J mice. J Neurol Sci. 1976 Nov;30(1):201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Melvold R. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J Immunol. 1984 Apr;132(4):1821–1825. [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., He C., Toth K. S., Zhang C. X., Lipton H. L. Three-dimensional structure of Theiler murine encephalomyelitis virus (BeAn strain). Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2409–2413. doi: 10.1073/pnas.89.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson D. H., Roos R. P., Arnason B. G. Comparison of agar gel electrophoresis and isoelectric focusing in multiple sclerosis and subacute sclerosing panencephalitis. Ann Neurol. 1981 Jan;9(1):34–41. doi: 10.1002/ana.410090107. [DOI] [PubMed] [Google Scholar]
- McAllister A., Tangy F., Aubert C., Brahic M. Genetic mapping of the ability of Theiler's virus to persist and demyelinate. J Virol. 1990 Sep;64(9):4252–4257. doi: 10.1128/jvi.64.9.4252-4257.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. L., Weigle W. O. Biological activities residing in the Fc region of immunoglobulin. Adv Immunol. 1987;40:61–134. doi: 10.1016/s0065-2776(08)60238-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Myers K. J., Sprent J., Dougherty J. P., Ron Y. Synergy between encephalitogenic T cells and myelin basic protein-specific antibodies in the induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1992 Nov;41(1):1–8. doi: 10.1016/0165-5728(92)90188-q. [DOI] [PubMed] [Google Scholar]
- Nitayaphan S., Toth M. M., Roos R. P. Localization of a neutralization site of Theiler's murine encephalomyelitis viruses. J Virol. 1985 Dec;56(3):887–895. doi: 10.1128/jvi.56.3.887-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra P. L., Faden H. S. Poliovirus vaccines: live or dead. J Pediatr. 1986 Jun;108(6):1031–1033. doi: 10.1016/s0022-3476(86)80957-x. [DOI] [PubMed] [Google Scholar]
- Ohara Y., Senkowski A., Fu J. L., Klaman L., Goodall J., Toth M., Roos R. P. Trypsin-sensitive neutralization site on VP1 of Theiler's murine encephalomyelitis viruses. J Virol. 1988 Sep;62(9):3527–3529. doi: 10.1128/jvi.62.9.3527-3529.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Stein S., Fu J. L., Stillman L., Klaman L., Roos R. P. Molecular cloning and sequence determination of DA strain of Theiler's murine encephalomyelitis viruses. Virology. 1988 May;164(1):245–255. doi: 10.1016/0042-6822(88)90642-3. [DOI] [PubMed] [Google Scholar]
- Peterson J. D., Waltenbaugh C., Miller S. D. IgG subclass responses to Theiler's murine encephalomyelitis virus infection and immunization suggest a dominant role for Th1 cells in susceptible mouse strains. Immunology. 1992 Apr;75(4):652–658. [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Calenoff M., Rozhon E., Lipton H. L. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987 May;61(5):1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., David C. S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985 Sep;135(3):2145–2148. [PubMed] [Google Scholar]
- Rodriguez M., Kenny J. J., Thiemann R. L., Woloschak G. E. Theiler's virus-induced demyelination in mice immunosuppressed with anti-IgM and in mice expressing the xid gene. Microb Pathog. 1990 Jan;8(1):23–35. doi: 10.1016/0882-4010(90)90005-b. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Nalefski E. A., Nitayaphan S., Variakojis R., Singh K. K. An isoelectric focusing overlay study of the humoral immune response in Theiler's virus demyelinating disease. J Neuroimmunol. 1987 Jan;13(3):305–314. doi: 10.1016/0165-5728(87)90066-x. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Stein S., Routbort M., Senkowski A., Bodwell T., Wollmann R. Theiler's murine encephalomyelitis virus neutralization escape mutants have a change in disease phenotype. J Virol. 1989 Oct;63(10):4469–4473. doi: 10.1128/jvi.63.10.4469-4473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. P., Cash E., Aubert C., Coutinho A. Role of the humoral immune response in resistance to Theiler's virus infection. J Virol. 1991 Jul;65(7):3895–3899. doi: 10.1128/jvi.65.7.3895-3899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangy F., McAllister A., Aubert C., Brahic M. Determinants of persistence and demyelination of the DA strain of Theiler's virus are found only in the VP1 gene. J Virol. 1991 Mar;65(3):1616–1618. doi: 10.1128/jvi.65.3.1616-1618.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M. SPONTANEOUS ENCEPHALOMYELITIS OF MICE--A NEW VIRUS DISEASE. Science. 1934 Aug 3;80(2066):122–122. doi: 10.1126/science.80.2066.122-a. [DOI] [PubMed] [Google Scholar]
- Thorpe R., Minor P. D., Mackay A., Schild G. C., Spitz M. Immunochemical studies of polioviruses: identification of immunoreactive virus capsid polypeptides. J Gen Virol. 1982 Dec;63(2):487–492. doi: 10.1099/0022-1317-63-2-487. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wychowski C., van der Werf S., Siffert O., Crainic R., Bruneau P., Girard M. A poliovirus type 1 neutralization epitope is located within amino acid residues 93 to 104 of viral capsid polypeptide VP1. EMBO J. 1983;2(11):2019–2024. doi: 10.1002/j.1460-2075.1983.tb01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A., Fujinami R. S. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J Virol. 1989 Apr;63(4):1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]