Abstract
Plant cell vacuoles may have either storage or degradative functions. Vegetative storage proteins (VSPs) are synthesized in response to wounding and to developmental switches that affect carbon and nitrogen sinks. Here we show that VSPs are stored in a unique type of vacuole that is derived from degradative central vacuoles coincident with insertion of a new tonoplast intrinsic protein (TIP), δ-TIP, into their membranes. This finding demonstrates a tight coupling between the presence of δ-TIP and acquisition of a specialized storage function and indicates that TIP isoforms may determine vacuole identity.
Uniquely, in contrast to yeast or mammalian cells, plant cells may contain separate vacuoles with protein storage and digestive functions (1, 2). It is not known how functionally distinct vacuoles are generated or maintained. Plant vacuole tonoplast membranes contain abundant tonoplast intrinsic proteins (TIPs) that may function as aquaporins (3), but the quantities present seem to be far in excess of what is required for water transport (4). Protein storage vacuoles (PSVs), containing seed-type storage proteins, are marked by the presence of α-TIP, and lytic or degradative vacuoles (LVs) are marked by the presence of γ-TIP (1, 2). These observations indicate that a specific TIP isoform correlates with a specific vacuole function (5). A further test of this hypothesis would require demonstration that a third functionally distinct vacuole carried a different TIP isoform. Here we define a third functionally distinct vacuole in plant cells and demonstrate that it is marked specifically by δ-TIP.
MATERIALS AND METHODS
Antibodies and Immunocytochemistry.
Anti-α-TIP protein antiserum (6) and VM23 antiserum to purified γ-TIP protein from radish root (7) were generously provided by M. Chrispeels (6) and M. Maeshima (7), respectively. Synthetic peptides were synthesized and antisera to the peptides coupled to keyhole limpet hemocyanin were generated by Quality Controlled Biochemicals, Hopkinton, MA. For antibody purification, the peptides were coupled via an amino-terminal Cys residue to SulfoLink agarose (Pierce) according to the manufacturer’s instructions, and peptide-specific antibodies were affinity-purified as described previously (8) for use in all procedures. Antisera to proteinase inhibitor I (Inh I) and II (Inh II) have been described previously (9–11). Fluorochrome-tagged secondary antibodies were purchased from Jackson ImmunoResearch. Plant tissues were fixed in either formaldehyde/acetic acid/ethyl alcohol or 3.7% paraformaldehyde, and paraffin-embedded sections were prepared as described (12). After removal of paraffin and rehydration, the sections were blocked as described (2). The double-label protocol to identify two different rabbit antibodies separately with immunofluorescence has been described (2). Briefly, the first primary antibody was completely covered by incubation with an excess of anti-rabbit F(ab′)2 fragment coupled to lissamine rhodamine before incubating with the second primary antibody and detection with a fluorescein isothiocyanate (FITC)-coupled secondary antibody. Slides were viewed and photographed under epifluorescence with an Olympus BX-50 microscope containing a multifilter set (no. 61002, Chroma Technology, Brattleboro VT) that permits simultaneous viewing of blue (462 nm), green (531 nm), and red (627 nm) emissions that are excited at 402 nm, 496 nm, and 571 nm, respectively. This filter set converts most background fluorescence from plant tissues, including that from chlorophyll, to gray, and thus facilitates identification of fluorescence resulting from specific antibodies. Photographic prints were scanned into a computer, and images representing individual red (lissamine rhodamine) and green (FITC) emissions were generated by using adobe photoshop software (Adobe Systems, Mountain View, CA).
Plant Material.
Growth of soybean plants and depodding to induce vegetative storage protein (VSP) accumulation has been described (13–15). Tomato, petunia, and tobacco plants were grown in Washington State University greenhouses, and other plant materials were purchased from a local supermarket. Tissue extracts for Western blot analyses (16) were prepared by homogenization at 25°C in 0.0625 M Tris⋅HCl, pH 6.8/2% SDS with a Polytron-type homogenizer (PRO250; PRO Scientific, Monroe, CT). After homogenization the extracts were allowed to sit for 1 hr at 25°C and then clarified by centrifugation at 12,000 × g.
RESULTS
Previous studies to identify specific TIP isoforms in vacuoles used antiserum to α-TIP, a single protein purified from bean seeds (6) and VM23 antiserum recognizing a purified γ-TIP protein purified from radish root (7). We noted that the carboxyl-terminal, cytoplasmic-tail sequences of the different TIPs varied such that it was possible to make antibodies specific for each: γ-TIP, THEQLPTTDY (barley, GenBank accession no. X80266; rice, D25534; Arabidopsis, Z34662 and X72581; radish, D84669); α-TIP, HQPLATEDY (Phaseolus, X62873) and HQPLAPEDY (Arabidopsis, X63551); DIP (17), HTPLPTSEDYA (Antirrhinum, X70417) and HAPLPTSEDYA (potato, U65700; tobacco, X54855); and δ-TIP, HVPLASADF (Arabidopsis, U39485). Accordingly, rabbits were immunized with the following peptides coupled to keyhole limpet hemocyanin: γ-TIP, CSRTHEQLPTTDY; α-TIP, C(aminohexanoic acid)HQPLAPEDY; DIP, CHTPLPTSEDYA; and δ-TIP, CHVPLASADF, and affinity-purified antibodies were tested on dot-blots containing the different peptides coupled to BSA. Each antibody preparation was specific, having at least a 100-fold-higher affinity for its corresponding peptide than for any of the other peptides (data not presented). Use of the anti-DIP peptide antibodies will be described elsewhere.
Anti-TIP Peptide Antibody Specificity.
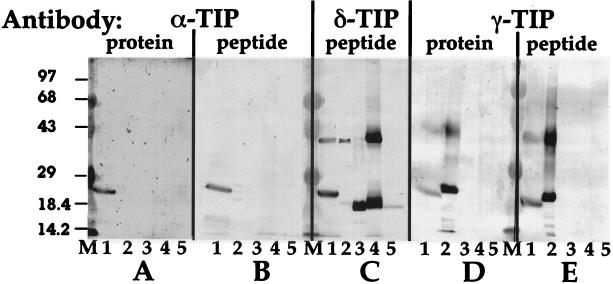
The specificity of the antibodies is demonstrated by the following results from Western blot analyses of different plant tissue extracts (Fig. 1). The anti-α-TIP protein antiserum (Fig. 1A) identified a band of the appropriate 26-kDa size in only the pea root tip extract (lane 1), and a similar pattern was obtained with our anti-α-TIP peptide antibodies (Fig. 1B). The VM23 anti-γ-TIP protein antibodies identified appropriate bands in both pea root tip and radish root (lane 2) (Fig. 1D), a pattern that was indistinguishable from that obtained with our anti-γ-TIP peptide antibodies (Fig. 1E). Therefore, results with the anti-peptide antibodies were indistinguishable from results in which antibodies that detect the entire α- and γ-TIP proteins were used. In immunofluorescence studies with pea and barley root tip cells (2), the anti-α- and anti-γ-TIP antibodies label separate α-TIP- and γ-TIP-containing vacuoles, respectively (G.-Y.J. and J.C.R., unpublished data); therefore, the ≈26-kDa band detected by the anti-γ-TIP antibodies in pea root tip extracts (Fig. 1 D and E, lane 1) is γ-TIP and does not represent cross-reactivity with α-TIP. [The ≈40-kDa bands represent TIP dimers that form when tissue extracts are heated during preparation of samples for electrophoresis (6, 7). Additionally, the variation in size of the ≈26-kDa TIP monomers is a result of proteolytic cleavage of the first transmembrane domain from the rest of the protein in certain plant tissues (18).] In contrast, the δ-TIP anti-peptide antibodies (Fig. 1C) identified specific TIP bands in pea root tips, radish root, flower petals from petunia (lane 3) and tobacco (lane 4), and potato tubers (lane 5). Neither α-TIP nor γ-TIP were detected in the extracts from flower petals and potato tuber. These findings led us to ask which functions might be associated with δ-TIP in potato tuber or flower petal vacuoles.
Figure 1.
Western blot analyses of plant tissue extracts with different anti-TIP antibodies. Membranes carrying 300 μg of protein from extracts of pea root tips (1), radish root (2), petunia flower petals (3), tobacco flower petals (4), and potato tuber (5) were tested with antibodies to α-TIP protein (A) (8) (1:1,000), α-TIP C-terminal peptide (B) (0.2 μg/ml), δ-TIP peptide (C) (0.95 μg/ml), γ-TIP protein (D) [VM23 antiserum (7), 1:1,000], and γ-TIP peptide (E) (0.24 μg/ml). M, molecular mass markers; sizes (in kDa) are indicated to the left.
Many plant species accumulate VSPs (19, 20). VSPs have physical characteristics that differ substantially from seed-type storage proteins present in PSVs and include proteins with diverse functions such as protease inhibitors, acid phosphatases, and lipoxygenases. VSPs may accumulate during vegetative growth, serving as temporary sites of carbon and nitrogen storage, and are mobilized as an energy source during seed development (19). In other circumstances, VSPs such as protease inhibitors accumulate in response to pathogen attack or wounding (9). Protease inhibitors are abundant in potato tubers, but initially are stored in leaves in plantlets until rhizomes form; then the inhibitors disappear from leaves and begin to accumulate in the developing tubers (21).
Immunofluorescence Localization.
We used immunofluorescence on paraffin-embedded tissue sections to assess localization of VSPs and their association with δ-TIP in several different tissues of potato, tomato, petunia, and soybean plants by using specific antibodies. In potato tuber (Fig. 2), strong labeling with the anti-δ-TIP antibodies (red) was present on central vacuole tonoplast in most cells (open arrow), and the same vacuoles were strongly labeled with antibodies to potato protease inhibitor I (21) (Inh I, green). Occasional cells had two distinct vacuoles: in addition to large vacuoles containing δ-TIP and Inh I (asterisk) there were smaller vacuoles (solid arrow) that did not label with either antibody. Additionally, in these cells, both δ-TIP and Inh I appeared to be present in numerous small organelles within the cytoplasm.
Figure 2.
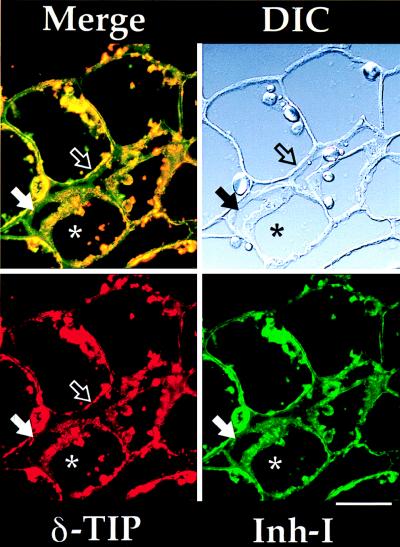
Immunofluorescence on potato tuber. A differential interference contrast (DIC) image of the potato tuber section is shown at the upper right. The double-label image (Merge) is in the upper left, with separated anti-δ-TIP (used at 19 μg/ml) (red) and anti-protease inhibitor I (Inh I) (1:100) (green) images at the bottom. Open arrow, central vacuole tonoplast staining with anti-δ-TIP, where Inh I is closely adherent; solid arrow, vacuole with tonoplast not staining with anti-δ-TIP and not containing Inh I in a cell with a larger vacuole (asterisk) labeled with the two antibodies. (Bar = 50 μm.)
Proteinase inhibitors in tomato leaves are induced by wounding or by methyl jasmonate treatment (10). We used antibodies to tomato proteinase inhibitor II (Inh II) (11) to compare its localization to that of δ-TIP in sections of leaves from plants treated with methyljasmonate for 2 days to induce accumulation of Inh II (Fig. 3 a and b). On Western blots, tomato leaf extracts have no detectable α- or γ-TIP, but have abundant δ-TIP (data not presented). Both Inh II (Fig. 3, green) and δ-TIP (red) antibodies strongly labeled epidermal cells (Fig. 3a), bundle sheath cells (not shown), and cells (Fig. 3b) with a tissue distribution typical of paraveinal mesophyll (PVM) as characterized in legumes. The two antibodies colocalized in small vacuoles (solid arrows) that were separate from the central vacuole in the cells; neither antigen appeared to be associated with the central vacuole. Controls used in all experiments support the specificity of patterns shown: (i) single labeling with each of the antibodies gave the same cell and organelle staining pattern (data not presented); and (ii) when sections labeled with the δ-TIP antibodies were incubated with the excess of rhodamine-conjugated anti-rabbit IgG F(ab′)2 secondary antibodies, washed, and then incubated with the FITC-conjugated anti-rabbit IgG secondary antibodies, no green labeling was obtained (data not presented). This demonstrates that the F(ab′)2 antibodies completely blocked all of the δ-TIP antibody sites so that FITC labeling in the sections shown in Fig. 3 must be a result of the presence of anti-proteinase inhibitor II antibodies at those sites.
Figure 3.
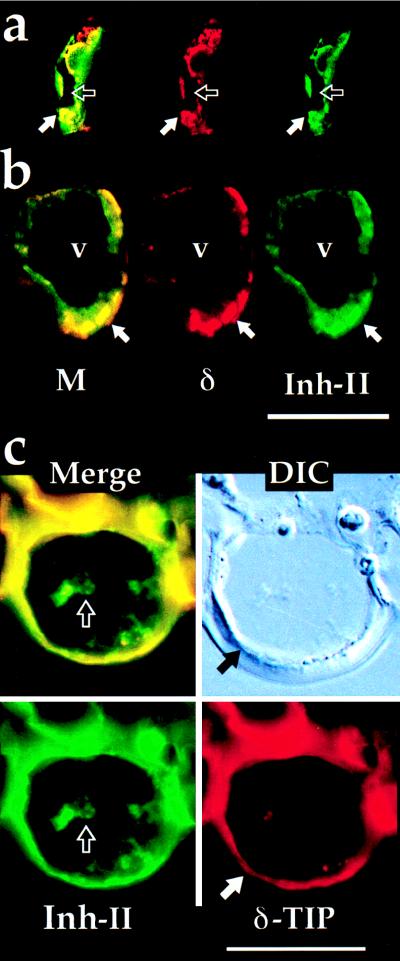
Immunofluorescence on tomato tissues. Anti-δ-TIP (δ) is in red and anti-Inh II (used at 1:100) is in green. M, double-label image. (a) Epidermal cell from leaf section. Solid arrow indicates small vacuole stained with both antibodies; open arrow indicates central vacuole. (b) PVM cell. Solid arrow as for a; v, central vacuole. (c) Epidermal cell from bottom surface of flower petal. Solid arrow indicates central vacuole tonoplast stained with anti-δ-TIP; open arrow indicates aggregated Inh II protein within lumen of central vacuole. (Bars = 25 μm.)
We found that tomato flower petals contain high concentrations of protease inhibitors I and II, 94 and 524 μg/ml of tissue extract, respectively. When sections of tomato flower petals were probed with antibodies against δ-TIP and Inh II, anti-δ-TIP (Fig. 3c, red) strongly labeled the tonoplast of the central vacuole (solid arrow) in all epidermal cells; double-labeling demonstrated that Inh II (Fig. 3c, green) was present in the contents of these vacuoles (open arrow). Anti-δ-TIP antibodies also labeled the central vacuole tonoplast of epidermal cells in petunia flower petals (Fig. 4a). As shown in Fig. 4b, pigment is present in the central vacuole of the upper epidermal cells.
Figure 4.
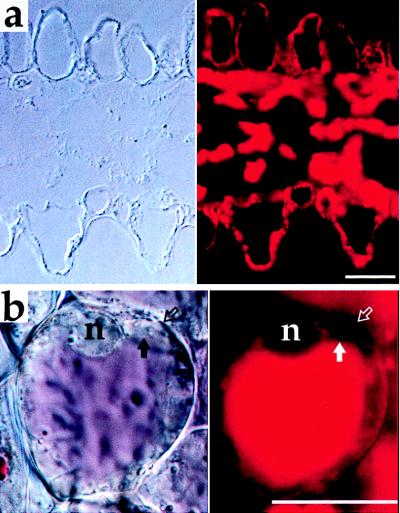
Immunofluorescence on petunia petal. (a) DIC (Left) and anti-δ-TIP labeling (Right). Upper and lower epidermal cells are shown at top and bottom, respectively. (b) Living cell from upper epidermis of petal. DIC image (Left) and endogenous fluorescence from vacuole contents (Right). Solid arrow, limit of vacuole; open arrow, cell wall; n = nucleus. (Bars = 25 μm.)
To test further the apparent association of δ-TIP and vacuoles containing VSPs, we utilized a soybean system in which deposition of VSPs in vacuoles of epidermal, bundle sheath, and PVM cells is induced by continuous depodding to remove the major nitrogen sink (13, 19, 22). Control plants were grown under identical conditions but were allowed to develop pods (13). Western blots demonstrated γ- and δ-TIP in leaf extracts of both control and depodded plants (data not presented). Double-label immunofluorescence of leaf sections with the two antibodies demonstrated that γ-TIP (Fig. 5a, red) labeled central vacuole tonoplast of epidermal (arrow), bundle sheath, and PVM (white dot) cells with no difference between control (Fig. 5a Left) and depodded (Fig. 5a Right) plants. In contrast, the pattern of δ-TIP labeling changed substantially in leaves from control versus depodded plants. In control (green, Fig. 5a Left), little labeling of epidermal (arrow) or PVM (white dot) was observed, while prominent labeling of those cells was observed in depodded plants (Fig. 5a Right). This is a specific difference because the maximum intensity of labeling of cells in the palisade layer (P) was similar in both control and depodded samples. The reproducibility of these observations was tested in a blinded manner by providing unlabeled slides with anti-γ- and anti-δ-TIP double-labeled sections to three independent observers; each observer accurately identified control and depodded samples. In Fig. 5b, the difference in δ-TIP abundance is compared in sections labeled with only that antibody. Spongy mesophyll cells stained with similar intensity in leaves from both controls and depodded plants. Clearly, however, there was abundant staining of the tonoplast in PVM (dot) and epidermal (asterisk) in leaves from depodded plants while very little staining of these cells was evident in the control plants. When similar sections were double-labeled with anti-δ-TIP antibodies and antibodies to soybean VSPs, the VSPs were present in PVM and epidermal cells (13, 15) whose vacuoles now carried δ-TIP in their tonoplast (data not presented). Thus, induction of VSP storage in soybean leaf cell vacuoles was accompanied by induction of δ-TIP expression in tonoplast of the same vacuoles while expression of γ-TIP was unchanged.
Figure 5.
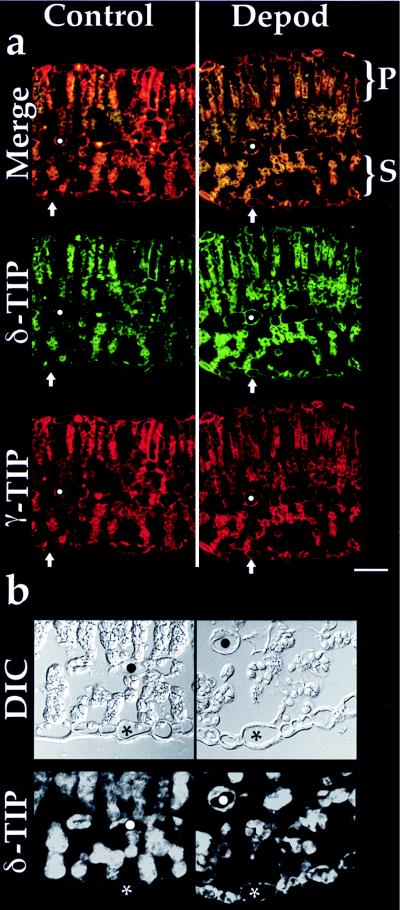
Immunofluorescence on soybean leaf. (a) Sections from leaves harvested at 4 weeks from control (Left) and continuously depodded (Right) plants are shown. Indicated are the palisade cell (P) and spongy mesophyll cell (S) layers; the white dot indicates a PVM cell, while the arrow indicates an epidermal cell on the lower surface of the leaf. Anti-δ-TIP is in green, and anti-γ-TIP (used at 8.8 μg/ml) is in red, with the double-label (Merge) image at the top. (Bar = 50 μm.) (b) Sections from leaves of control and depodded plants as in a. (Upper) DIC image. (Lower) Single label with anti-δ-TIP. ∗, Epidermal cell; •, PVM cell.
DISCUSSION
These data demonstrate that vacuoles whose tonoplast is labeled with anti-δ-TIP antibodies are used by plant cells to store pigments and VSPs, proteins synthesized in response to developmental and environmental cues. Central vacuoles in petunia and tomato flower petal epidermal cells contain pigments and protease inhibitors. Tonoplast in most central vacuoles in potato tubers labeled strongly with anti-δ-TIP antibodies, and only those vacuoles contained Inh I. Vacuoles in tomato leaf epidermal, bundle sheath, and PVM cells labeled with anti-δ-TIP antibodies and contained Inh II; these vacuoles were smaller and separate from central vacuoles in the same cells. In soybean leaves, central vacuoles marked by γ-TIP in their tonoplast acquired abundant δ-TIP in the same tonoplast when storage of VSPs in the vacuoles was induced by depodding. This finding demonstrates a tight coupling between the presence of δ-TIP and acquisition of a specialized vacuolar storage function by vacuoles that otherwise would have been assumed to have a lytic function given the presence of γ-TIP in their membranes. These results in aggregate define “delta vacuoles” (ΔVs) with δ-TIP in their tonoplast as specialized storage organelles that are structurally and functionally distinct from PSVs and LVs.
The pattern of labeling observed with anti-δ-TIP antibodies was unusual. Frequently large and numerous aggregates were present along the tonoplast and within the vacuole lumen (e.g., Fig. 2). In leaves from depodded soybean plants, these δ-TIP-containing aggregates frequently appeared almost to fill the vacuole lumen (e.g., Fig. 5b, cells adjacent to asterisk). This appearance indicates that formation of ΔVs may involve more than insertion of δ-TIP molecules into preexisting tonoplast. Perhaps these aggregates represent newly synthesized membrane with high concentrations of δ-TIP that accumulates as vesicles or strands within the vacuole lumen. It is likely that the aggregates provide a clue as to how the internal vacuole environment is changed to permit VSP accumulation.
Mechanisms for sorting soluble proteins to ΔVs have not been elucidated. The finding, however, that soybean vacuoles carrying the LV marker γ-TIP may be converted to ΔVs indicates that traffic of soluble proteins to at least some ΔVs probably involves a vacuolar-sorting receptor associated with the LV pathway (23, 24). This would explain why the vacuolar-sorting determinant of prosporamin (25), a sweet potato tuber protease inhibitor (26), interacts with that receptor (23, 27).
Acknowledgments
We thank Gregory Pearce for expert advice and assistance and Maarten Chrispeels and Masayoshi Maeshima for providing antisera. This research was funded by Grants DE-FG0397ER20277 from the Department of Energy and GM52427 from the National Institutes of Health to J.C.R., 95-03688 from the U.S. Department of Agriculture to H.D.G., and IBN 9601099 from the National Science Foundation to C.A.R.
ABBREVIATIONS
- VSP
vegetative storage protein
- TIP
tonoplast intrinsic protein
- PSV
protein storage vacuole
- LV
lytic vacuole
- Inh I
protease inhibitor I
- Inh II
protease inhibitor II
- PVM
paraveinal mesophyll
- ΔV
delta vacuole
- FITC
fluorescein isothiocyanate
- DIC
differential interference contrast
References
- 1. Hoh B, Hinz G, Jeong B-K, Robinson D G. J Cell Sci. 1995;108:299–310. doi: 10.1242/jcs.108.1.299. [DOI] [PubMed] [Google Scholar]
- 2.Paris N, Stanley C M, Jones R L, Rogers J C. Cell. 1996;85:563–572. doi: 10.1016/s0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- 3.Chrispeels M J, Maurel C. Plant Physiol. 1994;105:9–13. doi: 10.1104/pp.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higuchi, T., Hisada, H., Morishima, S., Okada, Y. & Maeshima, M. (1998) Plant Cell Physiol., in press. [DOI] [PubMed]
- 5.Neuhaus J-M, Rogers J C. Plant Mol Biol. 1998;38:127–144. [PubMed] [Google Scholar]
- 6.Johnson K D, Herman E M, Chrispeels M J. Plant Physiol. 1989;91:1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeshima M. Plant Physiol. 1992;98:1248–1254. doi: 10.1104/pp.98.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holwerda B C, Galvin N J, Baranski T J, Rogers J C. Plant Cell. 1990;2:1091–1106. doi: 10.1105/tpc.2.11.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan C A, Huisman O C. Nature (London) 1967;214:1047–1049. doi: 10.1038/2141047a0. [DOI] [PubMed] [Google Scholar]
- 10.Ryan C A. Curr Top Cell Regul. 1980;17:1–23. doi: 10.1016/b978-0-12-152817-1.50005-5. [DOI] [PubMed] [Google Scholar]
- 11.Graham J S, Pearce G, Merrywheather J, Titani K, Ericson L, Ryan C A. J Biol Chem. 1985;260:6561–6564. [PubMed] [Google Scholar]
- 12.Berlyn G P, Miksche J P. Botanical Microtechnique and Cytochemistry. Ames: Iowa State Univ. Press; 1976. [Google Scholar]
- 13.Tranbarger T J, Franceschi V R, Hildebrand D F, Grimes H D. Plant Cell. 1991;3:973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunker T W, Loetje D S, Stephenson L C, Creelman R A, Mullet J E, Grimes H D. Plant Cell. 1995;7:1319–1331. doi: 10.1105/tpc.7.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephenson L C, Bunker T W, Dubbs W E, Grimes H D. Plant Physiol. 1998;116:923–933. doi: 10.1104/pp.116.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers S W, Burks M, Rogers J C. Plant J. 1997;11:1359–1368. doi: 10.1046/j.1365-313x.1997.11061359.x. [DOI] [PubMed] [Google Scholar]
- 17.Culianez-Macia F A, Martin C. Plant J. 1993;4:717–725. doi: 10.1046/j.1365-313x.1993.04040717.x. [DOI] [PubMed] [Google Scholar]
- 18.Inoue K, Takeuchi Y, Nishimura M, Hara-Nishimura I. Plant Mol Biol. 1995;28:1089–1101. doi: 10.1007/BF00032669. [DOI] [PubMed] [Google Scholar]
- 19.Staswick P E. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:303–322. [Google Scholar]
- 20.Herman E M. In: Advances in Structural Biology. Malhotra S, editor. Greenwich, CT: JAI Press; 1994. pp. 243–283. [Google Scholar]
- 21.Ryan, C. A. & Pearce, G. (1998) Annu. Rev. Cell Dev. Biol. 14, in press. [DOI] [PubMed]
- 22.Staswick P E. Plant Physiol. 1988;87:250–254. doi: 10.1104/pp.87.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirsch T, Paris N, Butler J M, Beevers L, Rogers J C. Proc Natl Acad Sci USA. 1994;91:3403–3407. doi: 10.1073/pnas.91.8.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paris N, Rogers S W, Jiang L, Kirsch T, Beevers L, Phillips T E, Rogers J C. Plant Physiol. 1997;115:29–39. doi: 10.1104/pp.115.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura, K., Matsuoka, K., Mukumoto, F. & Watanabe, N. (1993) J. Exp. Bot. 44, Suppl., 331–338.
- 26.Yeh K-W, Chen J-C, Lin M-I, Chen Y-M, Lin C-Y. Plant Mol Biol. 1997;33:565–570. doi: 10.1023/a:1005764702510. [DOI] [PubMed] [Google Scholar]
- 27.Kirsch T, Saalbach G, Raikhel N V, Beevers L. Plant Physiol. 1996;111:469–474. doi: 10.1104/pp.111.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]