Abstract
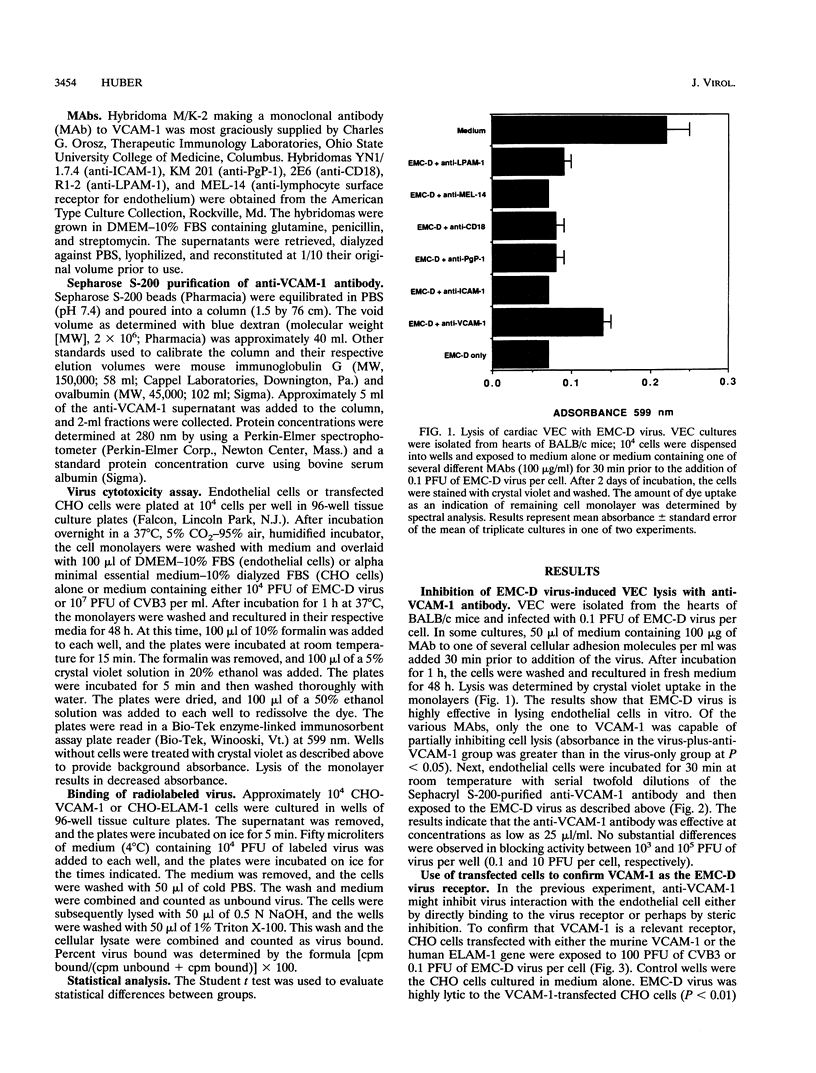
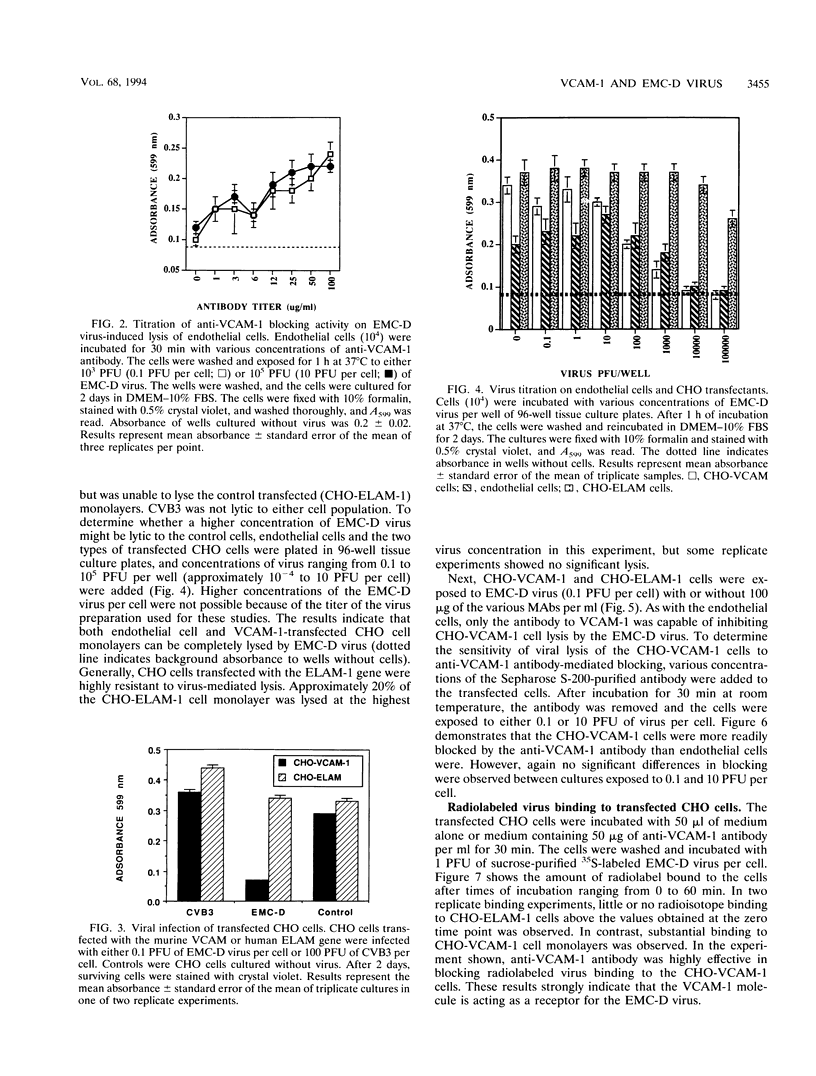
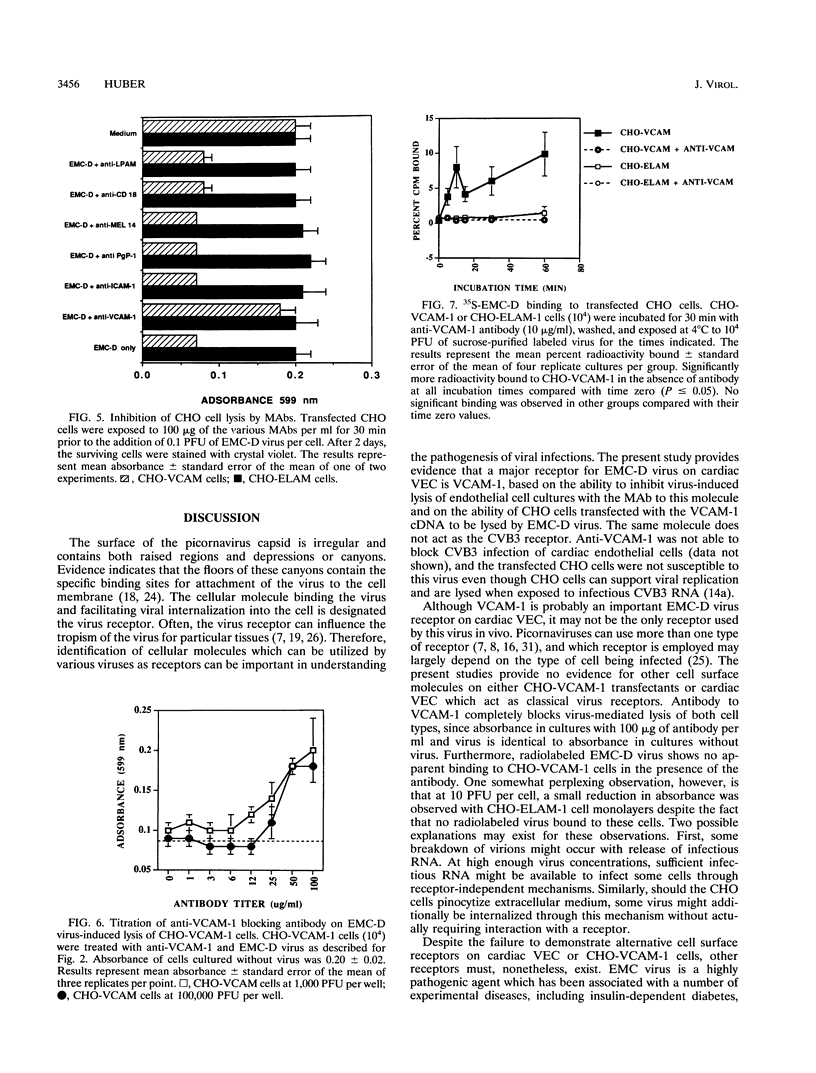
Murine VCAM-1 has been identified as a receptor for the D variant of encephalomyocarditis (EMC-D) virus on vascular endothelial cells from the heart. Monoclonal antibodies to VCAM-1 inhibited infection and lysis of endothelial cells with EMC-D virus. CHO cells transfected with the VCAM-1 gene were susceptible to EMC-D virus lysis, while control CHO cells transfected with the ELAM-1 gene were resistant. Similarly, 35S-labeled EMC-D virus bound to CHO-VCAM cells, and binding was inhibited with anti-VCAM-1 antibodies. Little or no radiolabeled virus bound to CHO-ELAM-1 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Lu W. C., Pardon E., Gumkowski F., Kaminska G., Kaminski M. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987 Mar 15;47(6):1492–1496. [PubMed] [Google Scholar]
- Barger M. T., Craighead J. E. Immunomodulation of encephalomyocarditis virus-induced disease in A/J mice. J Virol. 1991 May;65(5):2676–2681. doi: 10.1128/jvi.65.5.2676-2681.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni P. N., Nicolson G. L. Differential expression of cell surface glycoproteins on various organ-derived microvascular endothelia and endothelial cell cultures. J Cell Physiol. 1988 Sep;136(3):398–410. doi: 10.1002/jcp.1041360303. [DOI] [PubMed] [Google Scholar]
- Bergelson J. M., Shepley M. P., Chan B. M., Hemler M. E., Finberg R. W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science. 1992 Mar 27;255(5052):1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Craighead J. E., Huber S. A., Sriram S. Animal models of picornavirus-induced autoimmune disease: their possible relevance to human disease. Lab Invest. 1990 Oct;63(4):432–446. [PubMed] [Google Scholar]
- Crowell R. L., Field A. K., Schleif W. A., Long W. L., Colonno R. J., Mapoles J. E., Emini E. A. Monoclonal antibody that inhibits infection of HeLa and rhabdomyosarcoma cells by selected enteroviruses through receptor blockade. J Virol. 1986 Feb;57(2):438–445. doi: 10.1128/jvi.57.2.438-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Macarak E. J., MacGregor R. R., Wolfe J., Kefalides N. A. Virus infection of endothelial cells. J Infect Dis. 1981 Feb;143(2):266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Frizelle S., Schwarz J., Huber S. A., Leslie K. Evaluation of the effects of low molecular weight heparin on inflammation and collagen deposition in chronic coxsackievirus B3-induced myocarditis in A/J mice. Am J Pathol. 1992 Jul;141(1):203–209. [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Gumkowski F., Kaminska G., Kaminski M., Morrissey L. W., Auerbach R. Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels. 1987;24(1-2):11–23. [PubMed] [Google Scholar]
- Haynes M. K., Huber S. A., Craighead J. E. Helper-inducer T-lymphocytes mediate diabetes in EMC-infected BALB/c ByJ mice. Diabetes. 1987 Jul;36(7):877–881. doi: 10.2337/diab.36.7.877. [DOI] [PubMed] [Google Scholar]
- Hsu K. H., Lonberg-Holm K., Alstein B., Crowell R. L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988 May;62(5):1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Babu P. G., Craighead J. E. Genetic influences on the immunologic pathogenesis of encephalomyocarditis (EMC) virus-induced diabetes mellitus. Diabetes. 1985 Nov;34(11):1186–1190. doi: 10.2337/diab.34.11.1186. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Haisch C., Lodge P. A. Functional diversity in vascular endothelial cells: role in coxsackievirus tropism. J Virol. 1990 Sep;64(9):4516–4522. doi: 10.1128/jvi.64.9.4516-4522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., West D. C., Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36(1):57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Luo M., Vriend G., Kamer G., Minor I., Arnold E., Rossmann M. G., Boege U., Scraba D. G., Duke G. M., Palmenberg A. C. The atomic structure of Mengo virus at 3.0 A resolution. Science. 1987 Jan 9;235(4785):182–191. doi: 10.1126/science.3026048. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masinovsky B., Urdal D., Gallatin W. M. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990 Nov 1;145(9):2886–2895. [PubMed] [Google Scholar]
- Owman C., Hardebo J. E. Functional heterogeneity of the cerebrovascular endothelium. Brain Behav Evol. 1988;32(2):65–75. doi: 10.1159/000116534. [DOI] [PubMed] [Google Scholar]
- Pelletier R. P., Morgan C. J., Sedmak D. D., Miyake K., Kincade P. W., Ferguson R. M., Orosz C. G. Analysis of inflammatory endothelial changes, including VCAM-1 expression, in murine cardiac grafts. Transplantation. 1993 Feb;55(2):315–320. doi: 10.1097/00007890-199302000-00017. [DOI] [PubMed] [Google Scholar]
- Pelletier R. P., Ohye R. G., Vanbuskirk A., Sedmak D. D., Kincade P., Ferguson R. M., Orosz C. G. Importance of endothelial VCAM-1 for inflammatory leukocytic infiltration in vivo. J Immunol. 1992 Oct 1;149(7):2473–2481. [PubMed] [Google Scholar]
- Racaniello V. R. Cell receptors for picornaviruses. Curr Top Microbiol Immunol. 1990;161:1–22. doi: 10.1007/978-3-642-75602-3_1. [DOI] [PubMed] [Google Scholar]
- Reagan K. J., Goldberg B., Crowell R. L. Altered receptor specificity of coxsackievirus B3 after growth in rhabdomyosarcoma cells. J Virol. 1984 Mar;49(3):635–640. doi: 10.1128/jvi.49.3.635-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Tomassini J. E., Graham D., DeWitt C. M., Lineberger D. W., Rodkey J. A., Colonno R. J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. R., Beckstead J. H., Warnke R. A., Wood G. S. Endothelial cell phenotypic diversity. In situ demonstration of immunologic and enzymatic heterogeneity that correlates with specific morphologic subtypes. Am J Clin Pathol. 1987 May;87(5):569–575. doi: 10.1093/ajcp/87.5.569. [DOI] [PubMed] [Google Scholar]
- Weller A. H., Simpson K., Herzum M., Van Houten N., Huber S. A. Coxsackievirus-B3-induced myocarditis: virus receptor antibodies modulate myocarditis. J Immunol. 1989 Sep 15;143(6):1843–1850. [PubMed] [Google Scholar]



