Abstract
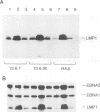
The Epstein-Barr virus in the Burkitt lymphoma-derived cell line Raji has a deletion in the EBNA3C gene. When Raji cells are allowed to grow to high density and most of the cells become growth arrested in the G1 phase of the cell cycle, the level of detectable latent membrane protein 1 (LMP1) is substantially reduced. After dilution of the cells with fresh growth medium, within 8 h, there is a large increase in LMP1 mRNA, and by 12 h, LMP1 is expressed to a high level (H. Boos, M. Stoehr, M. Sauter, and N. Mueller-Lantzch, J. Gen. Virol. 71:1811-1815, 1990). Here we show that in Raji cells which constitutively express a transfected EBNA3C gene, the down-regulation of LMP1 in growth-arrested cells does not take place. Furthermore, we show that in wild-type Raji cells, low-level LMP1 expression occurs when most of the cells are arrested at a point(s) early in G1 (or G0) when the product of the retinoblastoma gene, pRb, is hypophosphorylated. The dramatic synthesis of LMP1 coincides with the progression of these cells to late G1 when pRb becomes hyperphosphorylated. Thus, in Raji cells, the LMP1 gene is apparently regulated in a cell cycle- or proliferation-dependent manner, but when EBNA3C is present, sustained LMP1 expression occurs as it does in a lymphoblastoid cell line. EBNA3C appears to either relieve the apparent repression of LMP1 in cells progressing through early G1 or possibly alter the stage at which the cells growth arrest to one where they are permissive for LMP1 expression.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbot S. D., Rowe M., Cadwallader K., Ricksten A., Gordon J., Wang F., Rymo L., Rickinson A. B. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J Virol. 1990 May;64(5):2126–2134. doi: 10.1128/jvi.64.5.2126-2134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri C., Birkenbach M., Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991 Apr;181(2):595–608. doi: 10.1016/0042-6822(91)90893-g. [DOI] [PubMed] [Google Scholar]
- Allday M. J., Crawford D. H., Griffin B. E. Epstein-Barr virus latent gene expression during the initiation of B cell immortalization. J Gen Virol. 1989 Jul;70(Pt 7):1755–1764. doi: 10.1099/0022-1317-70-7-1755. [DOI] [PubMed] [Google Scholar]
- Allday M. J., Crawford D. H., Griffin B. E. Prediction and demonstration of a novel Epstein-Barr virus nuclear antigen. Nucleic Acids Res. 1988 May 25;16(10):4353–4367. doi: 10.1093/nar/16.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allday M. J., Crawford D. H., Thomas J. A. Epstein-Barr virus (EBV) nuclear antigen 6 induces expression of the EBV latent membrane protein and an activated phenotype in Raji cells. J Gen Virol. 1993 Mar;74(Pt 3):361–369. doi: 10.1099/0022-1317-74-3-361. [DOI] [PubMed] [Google Scholar]
- Allday M. J., MacGillivray A. J. Epstein-Barr virus nuclear antigen (EBNA): size polymorphism of EBNA 1. J Gen Virol. 1985 Jul;66(Pt 7):1595–1600. doi: 10.1099/0022-1317-66-7-1595. [DOI] [PubMed] [Google Scholar]
- Azim T., Allday M. J., Crawford D. H. Immortalization of Epstein-Barr virus-infected CD23-negative B lymphocytes by the addition of B cell growth factor. J Gen Virol. 1990 Mar;71(Pt 3):665–671. doi: 10.1099/0022-1317-71-3-665. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Blackwood E. M., Kretzner L., Eisenman R. N. Myc and Max function as a nucleoprotein complex. Curr Opin Genet Dev. 1992 Apr;2(2):227–235. doi: 10.1016/s0959-437x(05)80278-3. [DOI] [PubMed] [Google Scholar]
- Boos H., Berger R., Kuklik-Roos C., Iftner T., Mueller-Lantzsch N. Enhancement of Epstein-Barr virus membrane protein (LMP) expression by serum, TPA, or n-butyrate in latently infected Raji cells. Virology. 1987 Jul;159(1):161–165. doi: 10.1016/0042-6822(87)90360-6. [DOI] [PubMed] [Google Scholar]
- Boos H., Stoehr M., Sauter M., Mueller-Lantzsch N. Flow cytometric analysis of Epstein-Barr virus (EBV) latent membrane protein expression in EBV-infected Raji cells. J Gen Virol. 1990 Aug;71(Pt 8):1811–1815. doi: 10.1099/0022-1317-71-8-1811. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Mannick J., Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Schirm S., Bishop J. M. The MYC protein activates transcription of the alpha-prothymosin gene. EMBO J. 1991 Jan;10(1):133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus R., Fu H. L., Ernberg I., Finke J., Rowe M., Klein G., Falk K., Nilsson E., Yadav M., Busson P. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer. 1988 Sep 15;42(3):329–338. doi: 10.1002/ijc.2910420305. [DOI] [PubMed] [Google Scholar]
- Fåhraeus R., Jansson A., Ricksten A., Sjöblom A., Rymo L. Epstein-Barr virus-encoded nuclear antigen 2 activates the viral latent membrane protein promoter by modulating the activity of a negative regulatory element. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7390–7394. doi: 10.1073/pnas.87.19.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus R., Jansson A., Sjöblom A., Nilsson T., Klein G., Rymo L. Cell phenotype-dependent control of Epstein-Barr virus latent membrane protein 1 gene regulatory sequences. Virology. 1993 Jul;195(1):71–80. doi: 10.1006/viro.1993.1347. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Rabbitts T. H. Translocation joins c-myc and immunoglobulin gamma 1 genes in a Burkitt lymphoma revealing a third exon in the c-myc oncogene. Nature. 1983 Jul 14;304(5922):135–139. doi: 10.1038/304135a0. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B., Baichwal V. R. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J Virol. 1989 Jun;63(6):2469–2475. doi: 10.1128/jvi.63.6.2469-2475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hatfull G., Bankier A. T., Barrell B. G., Farrell P. J. Sequence analysis of Raji Epstein-Barr virus DNA. Virology. 1988 Jun;164(2):334–340. doi: 10.1016/0042-6822(88)90546-6. [DOI] [PubMed] [Google Scholar]
- Herbst H., Dallenbach F., Hummel M., Niedobitek G., Pileri S., Müller-Lantzsch N., Stein H. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4766–4770. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. E., Jr, Hensey C. E., Lee W. H. Retinoblastoma protein and the cell cycle. Curr Opin Genet Dev. 1993 Feb;3(1):55–62. doi: 10.1016/s0959-437x(05)80341-7. [DOI] [PubMed] [Google Scholar]
- Hotchin N. A., Allday M. J., Crawford D. H. Deregulated c-myc expression in Epstein-Barr-virus-immortalized B-cells induces altered growth properties and surface phenotype but not tumorigenicity. Int J Cancer. 1990 Mar 15;45(3):566–571. doi: 10.1002/ijc.2910450332. [DOI] [PubMed] [Google Scholar]
- Kaye K. M., Izumi K. M., Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Young L. S., Calender A., Gregory C. D., Rowe M., Lenoir G. M., Rickinson A. B. Different patterns of Epstein-Barr virus gene expression and of cytotoxic T-cell recognition in B-cell lines infected with transforming (B95.8) or nontransforming (P3HR1) virus strains. J Virol. 1988 Mar;62(3):894–901. doi: 10.1128/jvi.62.3.894-901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen G., Hamilton-Dutoit S. J., Rowe M., Young L. S. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet. 1991 Feb 9;337(8737):320–322. doi: 10.1016/0140-6736(91)90943-j. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Petti L., Sample J., Wang F., Kieff E. A fifth Epstein-Barr virus nuclear protein (EBNA3C) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Apr;62(4):1330–1338. doi: 10.1128/jvi.62.4.1330-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C., Howe J. G., Speck S. H., Miller G. Influence of Burkitt's lymphoma and primary B cells on latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J Virol. 1989 Apr;63(4):1531–1539. doi: 10.1128/jvi.63.4.1531-1539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N. S., Burke L. C., Bybee A., Linch D. C. The phosphorylation state of the retinoblastoma (RB) protein in G0/G1 is dependent on growth status. Oncogene. 1991 Feb;6(2):317–322. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B., Robertson E., Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J Virol. 1993 Apr;67(4):2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Walls E. V., Doyle M. G., Patel K. K., Allday M. J., Catovsky D., Crawford D. H. Activation and immortalization of leukaemic B cells by Epstein-Barr virus. Int J Cancer. 1989 Nov 15;44(5):846–853. doi: 10.1002/ijc.2910440517. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Tsang S. F., Kurilla M. G., Cohen J. I., Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990 Jul;64(7):3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Miller G. Recovery of Epstein-Barr virus from nonproducer neonatal human lymphoid cell transformants. Virology. 1979 Jun;95(2):351–358. doi: 10.1016/0042-6822(79)90490-2. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]