Abstract
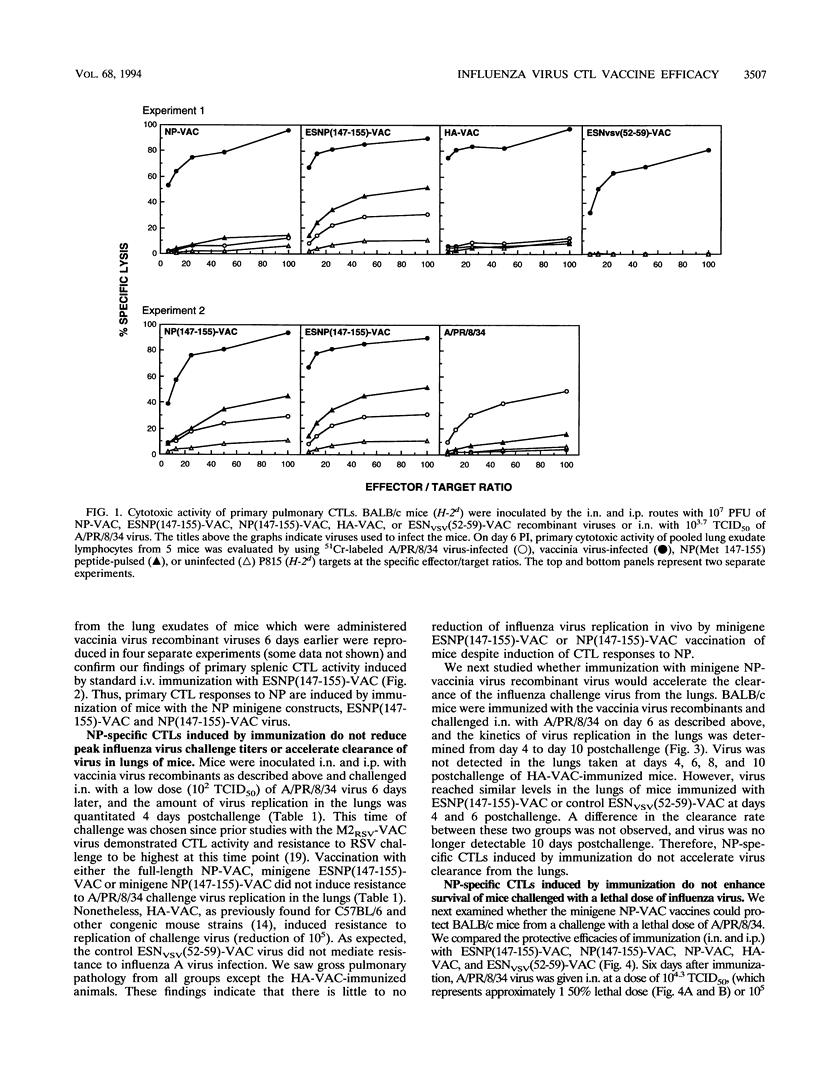
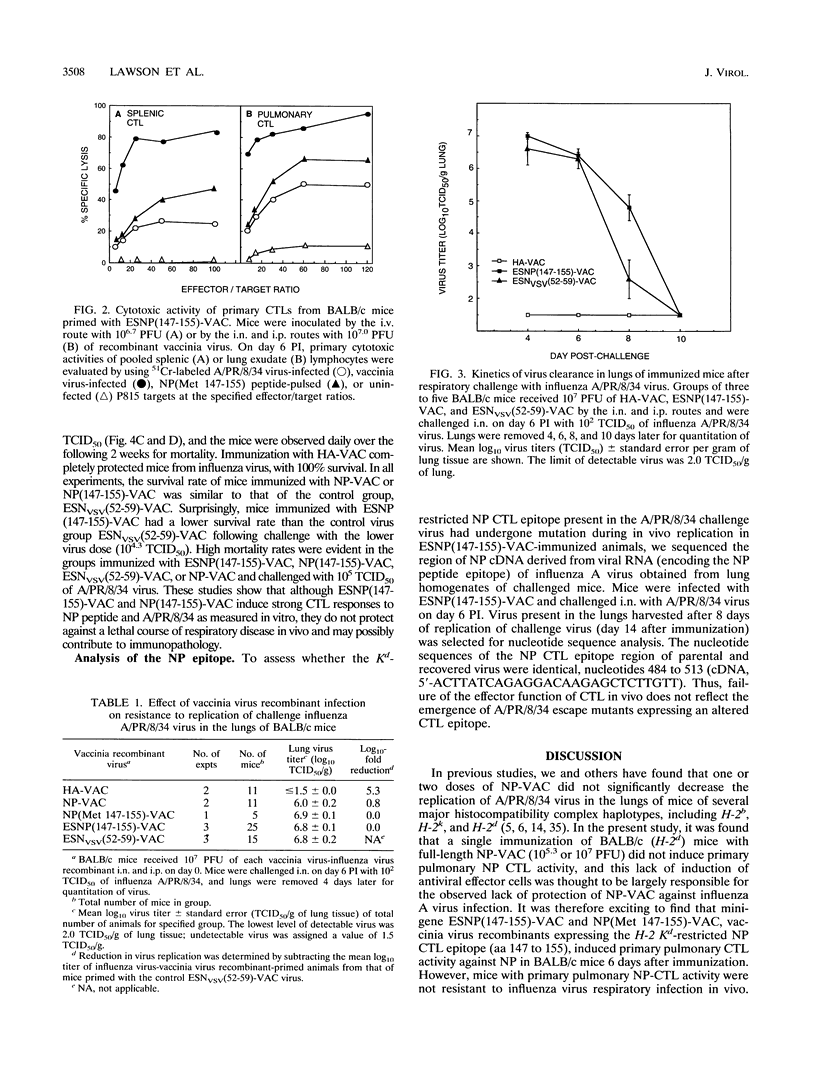
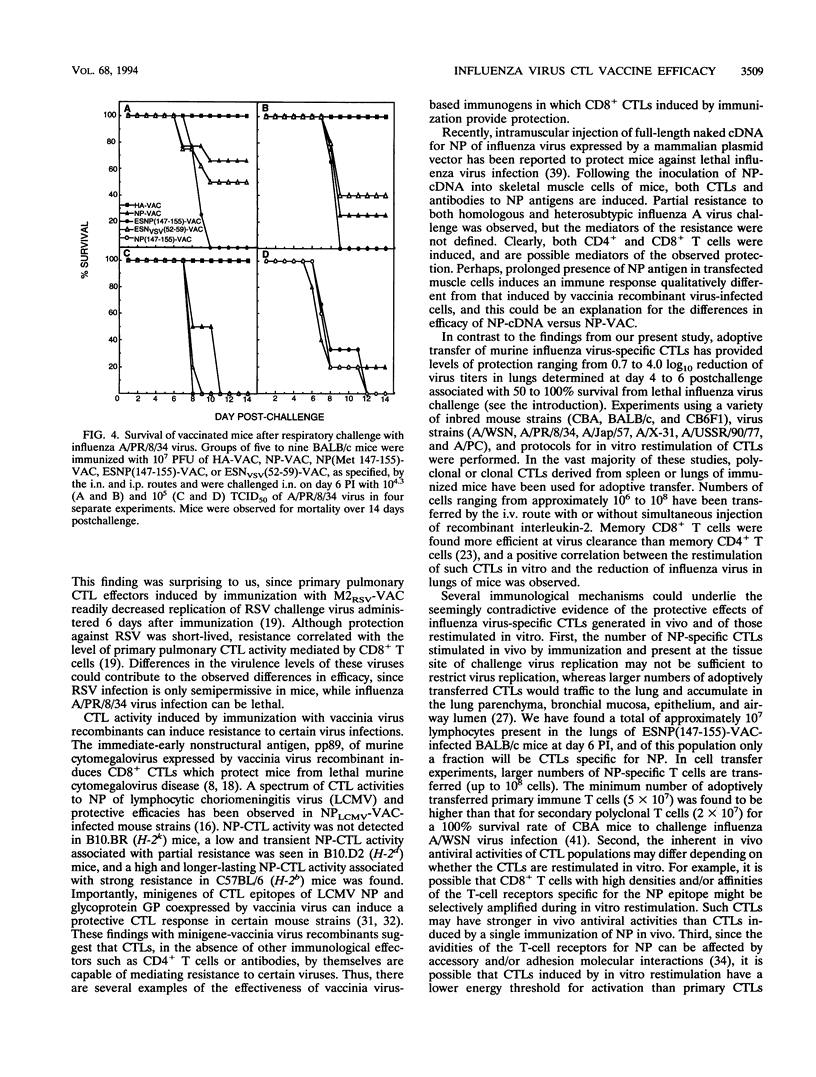
The nucleoprotein (NP) of influenza A virus is the dominant antigen recognized by influenza virus-specific cytotoxic T lymphocytes (CTLs), and adoptive transfer of NP-specific CTLs protects mice from influenza A virus infection. BALB/c mouse cells (H-2d) recognize a single Kd-restricted CTL epitope of NP consisting of amino acids 147 to 155. In the present study, mice were immunized with various vaccinia virus recombinant viruses to examine the effect of the induction of primary pulmonary CTLs on resistance to challenge with influenza A/Puerto Rico/8/34 virus. The minigene ESNP(147-155)-VAC construct, composed of a signal sequence from the adenovirus E3/19K glycoprotein (designated ES) and expressing the 9-amino-acid NP natural determinant (amino acids 147 to 155) preceded by an alanine residue, a similar minigene NP(Met 147-155)-VAC lacking ES, and a full-length NP-VAC recombinant of influenza virus were analyzed. The two minigene NP-VAC recombinants induced a greater primary pulmonary CTL response than the full-length NP-VAC recombinant. However, NP-specific CTLs induced by immunization with ESNP(147-155)-VAC did not decrease peak virus titer or accelerate clearance of virus in the lungs of mice challenged intranasally with A/PR/8/34. Furthermore, NP-specific CTLs induced by immunization did not protect mice challenged intranasally with a lethal dose of A/PR/8/34. Sequence analysis of the NP CTL epitope of A/PR/8/34 challenge virus obtained from lungs after 8 days of replication in ESNP(147-155)-VAC-immunized mice showed identity with that of the input virus, demonstrating that an escape mutant had not emerged during replication in vivo. Thus, in contrast to adoptively transferred CTLs, pulmonary NP-specific CTLs induced by recombinant vaccinia virus immunization do not have protective in vivo antiviral activity against influenza virus infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Anderson K., Cresswell P., Gammon M., Hermes J., Williamson A., Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J Exp Med. 1991 Aug 1;174(2):489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew M. E., Coupar B. E., Ada G. L., Boyle D. B. Cell-mediated immune responses to influenza virus antigens expressed by vaccinia virus recombinants. Microb Pathog. 1986 Oct;1(5):443–452. doi: 10.1016/0882-4010(86)90006-9. [DOI] [PubMed] [Google Scholar]
- Andrew M. E., Coupar B. E., Boyle D. B., Ada G. L. The roles of influenza virus haemagglutinin and nucleoprotein in protection: analysis using vaccinia virus recombinants. Scand J Immunol. 1987 Jan;25(1):21–28. doi: 10.1111/j.1365-3083.1987.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Andrew M. E., Coupar B. E. Efficacy of influenza haemagglutinin and nucleoprotein as protective antigens against influenza virus infection in mice. Scand J Immunol. 1988 Jul;28(1):81–85. doi: 10.1111/j.1365-3083.1988.tb02418.x. [DOI] [PubMed] [Google Scholar]
- Armerding D., Liehl E. Induction of homotypic and heterotypic T- and B-cell immunity with influenza A virus in mice. Cell Immunol. 1981 May 1;60(1):119–135. doi: 10.1016/0008-8749(81)90253-7. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- Del Val M., Schlicht H. J., Ruppert T., Reddehase M. J., Koszinowski U. H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991 Sep 20;66(6):1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Boyle D. B., Coupar B. E., Andrew M. E. Recombinant vaccinia viruses and the development of immunization strategies using influenza virus. J Infect Dis. 1989 Jun;159(6):1119–1122. doi: 10.1093/infdis/159.6.1119. [DOI] [PubMed] [Google Scholar]
- Eisenlohr L. C., Bacik I., Bennink J. R., Bernstein K., Yewdell J. W. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell. 1992 Dec 11;71(6):963–972. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- Eisenlohr L. C., Yewdell J. W., Bennink J. R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992 Feb 1;175(2):481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A., Itamura S., Iinuma H., Funahashi S., Shida H., Koide F., Nerome K., Oya A. Homotypic and heterotypic protection against influenza virus infection in mice by recombinant vaccinia virus expressing the haemagglutinin or nucleoprotein of influenza virus. J Gen Virol. 1991 Mar;72(Pt 3):699–703. doi: 10.1099/0022-1317-72-3-699. [DOI] [PubMed] [Google Scholar]
- Epstein S. L., Misplon J. A., Lawson C. M., Subbarao E. K., Connors M., Murphy B. R. Beta 2-microglobulin-deficient mice can be protected against influenza A infection by vaccination with vaccinia-influenza recombinants expressing hemagglutinin and neuraminidase. J Immunol. 1993 Jun 15;150(12):5484–5493. [PubMed] [Google Scholar]
- Flexner C., Murphy B. R., Rooney J. F., Wohlenberg C., Yuferov V., Notkins A. L., Moss B. Successful vaccination with a polyvalent live vector despite existing immunity to an expressed antigen. Nature. 1988 Sep 15;335(6187):259–262. doi: 10.1038/335259a0. [DOI] [PubMed] [Google Scholar]
- Hany M., Oehen S., Schulz M., Hengartner H., Mackett M., Bishop D. H., Overton H., Zinkernagel R. M. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur J Immunol. 1989 Mar;19(3):417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- Henderson R. A., Michel H., Sakaguchi K., Shabanowitz J., Appella E., Hunt D. F., Engelhard V. H. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992 Mar 6;255(5049):1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- Jonjić S., del Val M., Keil G. M., Reddehase M. J., Koszinowski U. H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J Virol. 1988 May;62(5):1653–1658. doi: 10.1128/jvi.62.5.1653-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Connors M., Firestone C. Y., Morse H. C., 3rd, Murphy B. R. The cytolytic activity of pulmonary CD8+ lymphocytes, induced by infection with a vaccinia virus recombinant expressing the M2 protein of respiratory syncytial virus (RSV), correlates with resistance to RSV infection in mice. J Virol. 1993 Feb;67(2):1044–1049. doi: 10.1128/jvi.67.2.1044-1049.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano K., Scott M., Young J. F., Ennis F. A. Active immunization against virus infections due to antigenic drift by induction of crossreactive cytotoxic T lymphocytes. J Exp Med. 1989 Apr 1;169(4):1361–1371. doi: 10.1084/jem.169.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano K., Tamura M., Ennis F. A. Cross-reactive protection against influenza A virus infections by an NS1-specific CTL clone. Virology. 1990 Sep;178(1):174–179. doi: 10.1016/0042-6822(90)90391-4. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., Subbarao E. K., Murphy B. R. Nucleotide sequence changes in the polymerase basic protein 2 gene of temperature-sensitive mutants of influenza A virus. Virology. 1992 Nov;191(1):506–510. doi: 10.1016/0042-6822(92)90221-a. [DOI] [PubMed] [Google Scholar]
- Lightman S., Cobbold S., Waldmann H., Askonas B. A. Do L3T4+ T cells act as effector cells in protection against influenza virus infection. Immunology. 1987 Sep;62(1):139–144. [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C. D., Taylor P. M., Askonas B. A. Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology. 1989 Jul;67(3):375–381. [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Lukacher A. E., Braciale V. L., Braciale T. J., Bienenstock J. Characterization and in vivo distribution of influenza-virus-specific T-lymphocytes in the murine respiratory tract. Am Rev Respir Dis. 1987 Jan;135(1):245–249. doi: 10.1164/arrd.1987.135.1.245. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Moskophidis D., Lechner F., Pircher H., Zinkernagel R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993 Apr 22;362(6422):758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Nonacs R., Humborg C., Tam J. P., Steinman R. M. Mechanisms of mouse spleen dendritic cell function in the generation of influenza-specific, cytolytic T lymphocytes. J Exp Med. 1992 Aug 1;176(2):519–529. doi: 10.1084/jem.176.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Eddleston M., de la Torre J. C., McKee T., Whitton J. L. Vaccination to prevent persistent viral infection. J Virol. 1993 Jul;67(7):4372–4378. doi: 10.1128/jvi.67.7.4372-4378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Geckeler R., Lewicki H., Whitton J. L. A common antiviral cytotoxic T-lymphocyte epitope for diverse major histocompatibility complex haplotypes: implications for vaccination. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2752–2755. doi: 10.1073/pnas.89.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. H., Betts R. F., DeBorde D., Tierney E. L., Clements M. L., Herrington D., Sears S. D., Dolin R., Maassab H. F., Murphy B. R. Four viral genes independently contribute to attenuation of live influenza A/Ann Arbor/6/60 (H2N2) cold-adapted reassortant virus vaccines. J Virol. 1988 Feb;62(2):488–495. doi: 10.1128/jvi.62.2.488-495.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser D. E., Kyburz D., Stübi U., Hengartner H., Zinkernagel R. M. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J Immunol. 1992 Aug 1;149(3):972–980. [PubMed] [Google Scholar]
- Stitz L., Schmitz C., Binder D., Zinkernagel R., Paoletti E., Becht H. Characterization and immunological properties of influenza A virus nucleoprotein (NP): cell-associated NP isolated from infected cells or viral NP expressed by vaccinia recombinant virus do not confer protection. J Gen Virol. 1990 May;71(Pt 5):1169–1179. doi: 10.1099/0022-1317-71-5-1169. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Askonas B. A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986 Jul;58(3):417–420. [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Donnelly J. J., Parker S. E., Rhodes G. H., Felgner P. L., Dwarki V. J., Gromkowski S. H., Deck R. R., DeWitt C. M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Kawaoka Y., Taylor J., Weinberg R., Paoletti E. Efficacy of nucleoprotein and haemagglutinin antigens expressed in fowlpox virus as vaccine for influenza in chickens. Vaccine. 1991 May;9(5):303–308. doi: 10.1016/0264-410x(91)90055-b. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L. The recovery of mice from influenza virus infection: adoptive transfer of immunity with immune T lymphocytes. Scand J Immunol. 1978;7(5):389–397. doi: 10.1111/j.1365-3083.1978.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]


