Abstract
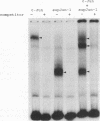
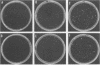
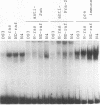
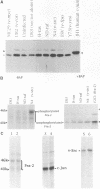
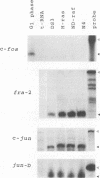
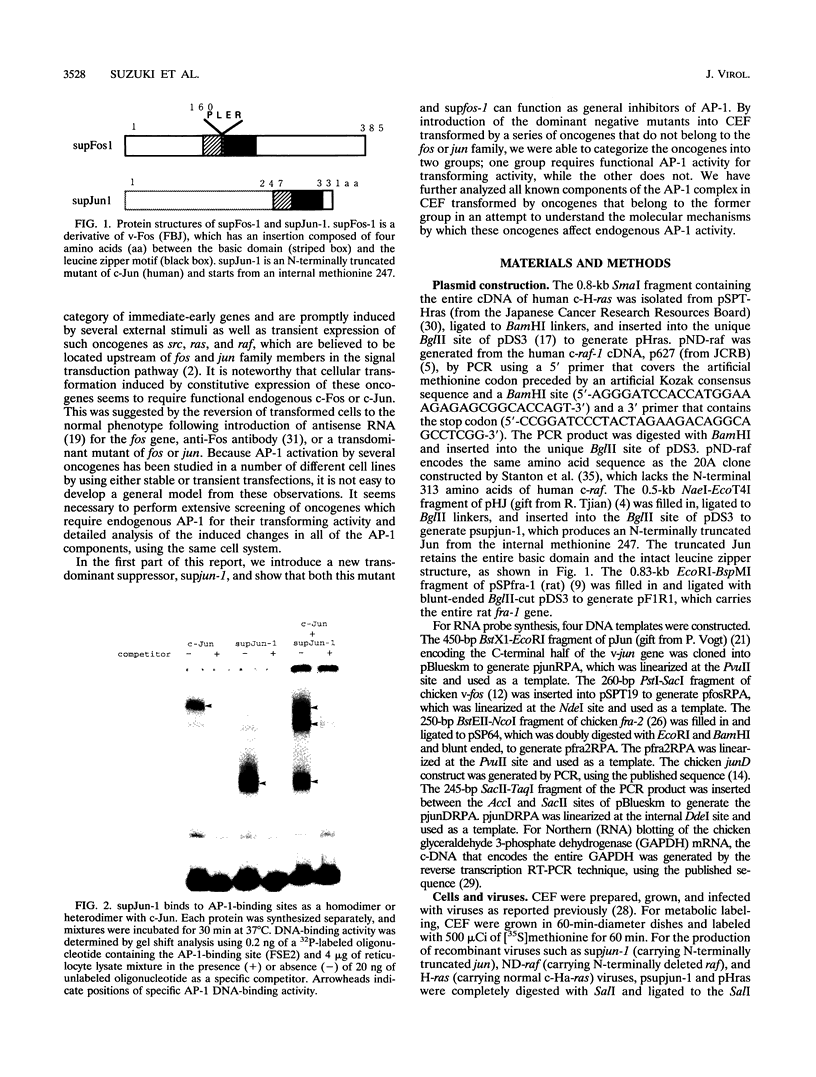
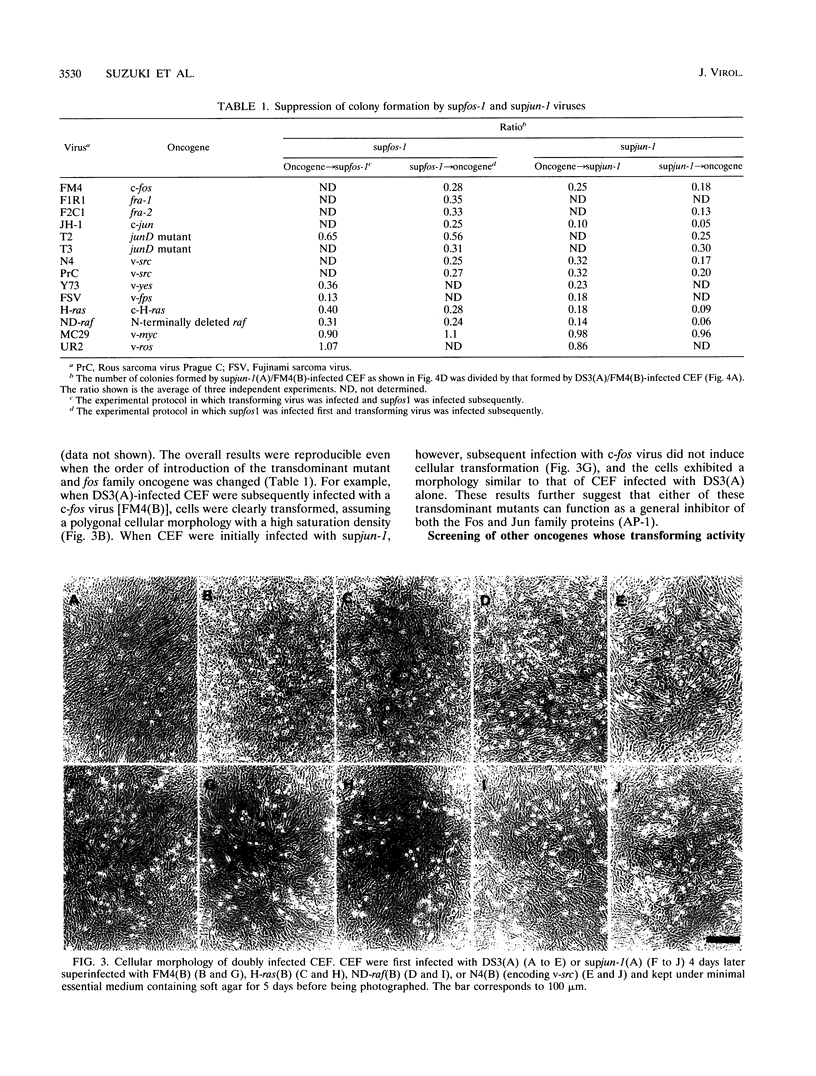
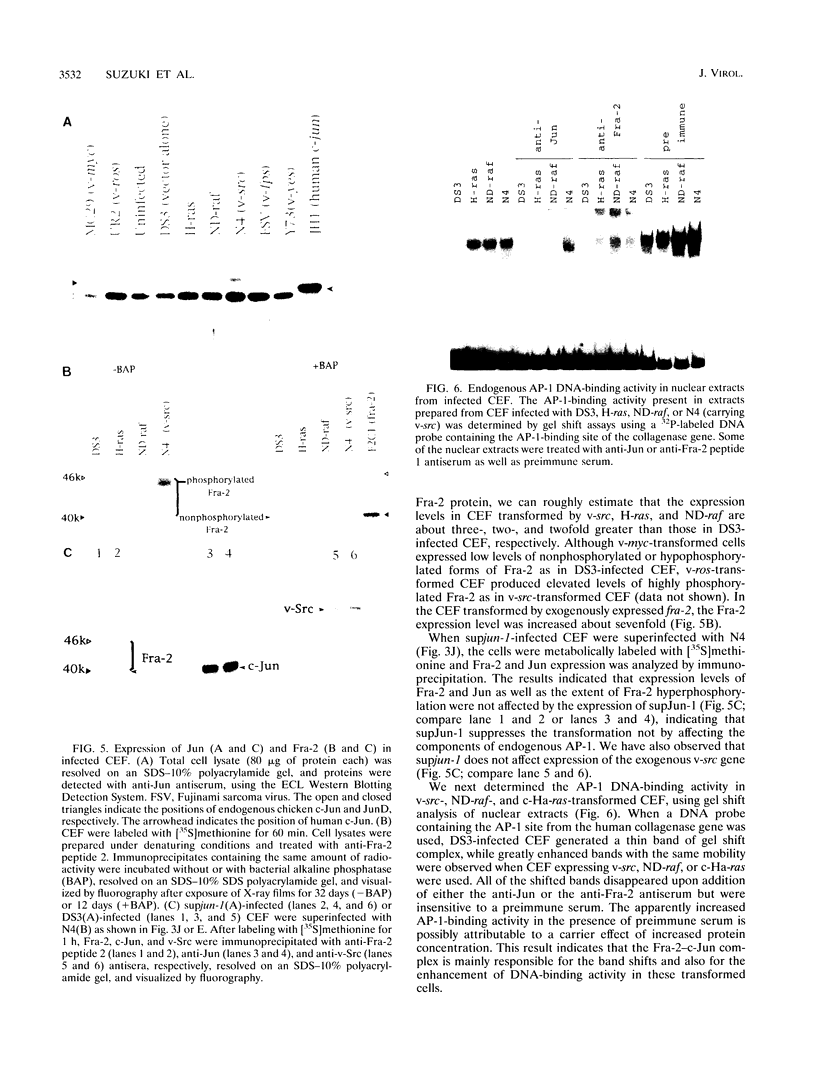
To understand the role of endogenous AP-1 activity in cellular transformation induced by oncogenes, we have made use of a fos mutant (supfos-1) and a jun mutant (supjun-1), either of which can function as a transdominant inhibitor of AP-1-mediated transcriptional regulation. Chicken embryo fibroblasts (CEF) infected with a series of transforming retroviruses were doubly infected with retrovirus carrying supfos-1 or supjun-1, and suppression of cellular transformation was monitored in terms of reversion to normal cellular morphology or acquisition of anchorage-dependent growth. Cellular transformation induced by several exogenously expressed transforming genes of the fos or jun family was efficiently suppressed, as expected. CEF transformed by v-src, v-yes, v-fps, c-Ha-ras, and N-terminally truncated c-raf were also induced to revert to the normal phenotype by these transdominant mutants, suggesting that functional transcription factor AP-1 activity is essential for the cellular transformation induced by these oncogenes. The suppression is not attributable to nonspecific inhibition of cellular proliferation, because CEF transformed by v-ros or v-myc were not induced to revert to the normal phenotype. We next analyzed changes in all known components of chicken AP-1 induced by v-src, c-Ha-ras, or activated c-raf transformation. The levels of both Fra-2 and c-Jun expression were elevated two- to fourfold, and hyperphosphorylation of Fra-2 was also observed. We further showed that Fra-2-c-Jun heterodimer is mainly responsible for the elevated AP-1 DNA-binding activity in these transformed cells, and we propose that this heterodimer play a crucial role in the transformation induced by these oncogenes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991 Jul;11(7):3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Kerby S. B., Sutrave P., Gunnell M. A., Mark G., Rapp U. R. Structure and biological activity of human homologs of the raf/mil oncogene. Mol Cell Biol. 1985 Jun;5(6):1400–1407. doi: 10.1128/mcb.5.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Alani R., Preis L. H., Szabo E., Birrer M. J. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993 Apr;8(4):877–886. [PubMed] [Google Scholar]
- Catling A. D., Wyke J. A., Frame M. C. Mitogenesis of quiescent chick fibroblasts by v-Src: dependence on events at the membrane leading to early changes in AP-1. Oncogene. 1993 Jul;8(7):1875–1886. [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Van Beveren C., Verma I. M. Viral and cellular fos proteins are complexed with a 39,000-dalton cellular protein. Mol Cell Biol. 1985 Jan;5(1):167–172. doi: 10.1128/mcb.5.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K. T., Ashida K., Nishina H., Iba H., Miyajima N., Nishizawa M., Kawai S. The chicken c-fos gene: cloning and nucleotide sequence analysis. J Virol. 1987 Dec;61(12):4012–4018. doi: 10.1128/jvi.61.12.4012-4018.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger-Schnarr M., Benusiglio E., Schnarr M., Sassone-Corsi P. Transformation and transactivation suppressor activity of the c-Jun leucine zipper fused to a bacterial repressor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4236–4239. doi: 10.1073/pnas.89.10.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl M., Hutchins J. T., Vogt P. K. The chicken junD gene and its product. Oncogene. 1991 Sep;6(9):1623–1631. [PubMed] [Google Scholar]
- Hartl M., Vogt P. K. A rearranged junD transforms chicken embryo fibroblasts. Cell Growth Differ. 1992 Dec;3(12):909–918. [PubMed] [Google Scholar]
- Hirai S. I., Ryseck R. P., Mechta F., Bravo R., Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989 May;8(5):1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Shindo Y., Nishina H., Yoshida T. Transforming potential and growth stimulating activity of the v-fos and c-fos genes carried by avian retrovirus vectors. Oncogene Res. 1988;2(2):121–133. [PubMed] [Google Scholar]
- Kameda T., Akahori A., Sonobe M. H., Suzuki T., Endo T., Iba H. JunD mutants with spontaneously acquired transforming potential have enhanced transactivating activity in combination with Fra-2. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9369–9373. doi: 10.1073/pnas.90.20.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledwith B. J., Manam S., Kraynak A. R., Nichols W. W., Bradley M. O. Antisense-fos RNA causes partial reversion of the transformed phenotypes induced by the c-Ha-ras oncogene. Mol Cell Biol. 1990 Apr;10(4):1545–1555. doi: 10.1128/mcb.10.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A., Yancheva N., Wasylyk B. Transformation suppressor activity of a Jun transcription factor lacking its activation domain. Nature. 1991 Aug 15;352(6336):635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- Maki Y., Bos T. J., Davis C., Starbuck M., Vogt P. K. Avian sarcoma virus 17 carries the jun oncogene. Proc Natl Acad Sci U S A. 1987 May;84(9):2848–2852. doi: 10.1073/pnas.84.9.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Tokuhara M., Konuma Y., Nomura N., Ishizaki R. Isolation of human fos-related genes and their expression during monocyte-macrophage differentiation. Oncogene. 1990 Mar;5(3):249–255. [PubMed] [Google Scholar]
- Métivier C., Piu F., Pfarr C. M., Yaniv M., Loiseau L., Castellazzi M. In vitro transforming capacities of mouse c-jun:junD chimeric genes. Oncogene. 1993 Aug;8(8):2311–2315. [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Vogt P. K. The avian cellular homolog of the oncogene jun. Oncogene. 1988 Dec;3(6):659–663. [PubMed] [Google Scholar]
- Nishina H., Sato H., Suzuki T., Sato M., Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci U S A. 1990 May;87(9):3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H., Suzuki T., Yoshida T., Hashimoto Y., Curran T., Iba H. Inhibition of jun transformation by a mutated fos gene: design of an anti-oncogene. Oncogene. 1991 Sep;6(9):1491–1497. [PubMed] [Google Scholar]
- Panabières F., Piechaczyk M., Rainer B., Dani C., Fort P., Riaad S., Marty L., Imbach J. L., Jeanteur P., Blanchard J. M. Complete nucleotide sequence of the messenger RNA coding for chicken muscle glyceraldehyde-3-phosphate dehydrogenase. Biochem Biophys Res Commun. 1984 Feb 14;118(3):767–773. doi: 10.1016/0006-291x(84)91461-x. [DOI] [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991 Dec 12;354(6353):494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Stanton V. P., Jr, Nichols D. W., Laudano A. P., Cooper G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989 Feb;9(2):639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Hashimoto Y., Okuno H., Sato H., Nishina H., Iba H. High-level expression of human c-jun gene causes cellular transformation of chicken embryo fibroblasts. Jpn J Cancer Res. 1991 Jan;82(1):58–64. doi: 10.1111/j.1349-7006.1991.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Okuno H., Yoshida T., Endo T., Nishina H., Iba H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 1991 Oct 25;19(20):5537–5542. doi: 10.1093/nar/19.20.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M., Lucibello F. C., Müller R. Inhibition of Fos- and Ras-induced transformation by mutant Fos proteins with structural alterations in functionally different domains. Oncogene. 1992 May;7(5):859–867. [PubMed] [Google Scholar]
- Yoshida T., Sato H., Iba H. Transcription of fra-2 mRNA and phosphorylation of Fra-2 protein are stimulated by serum. Biochem Biophys Res Commun. 1991 Jan 31;174(2):934–939. doi: 10.1016/0006-291x(91)91508-a. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Shindo Y., Ohta K., Iba H. Identification of a small region of the v-fos gene product that is sufficient for transforming potential and growth-stimulating activity. Oncogene Res. 1989;5(2):79–89. [PubMed] [Google Scholar]
- Yoshida T., Suzuki T., Sato H., Nishina H., Iba H. Analysis of fra-2 gene expression. Nucleic Acids Res. 1993 Jun 11;21(11):2715–2721. doi: 10.1093/nar/21.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]