Abstract
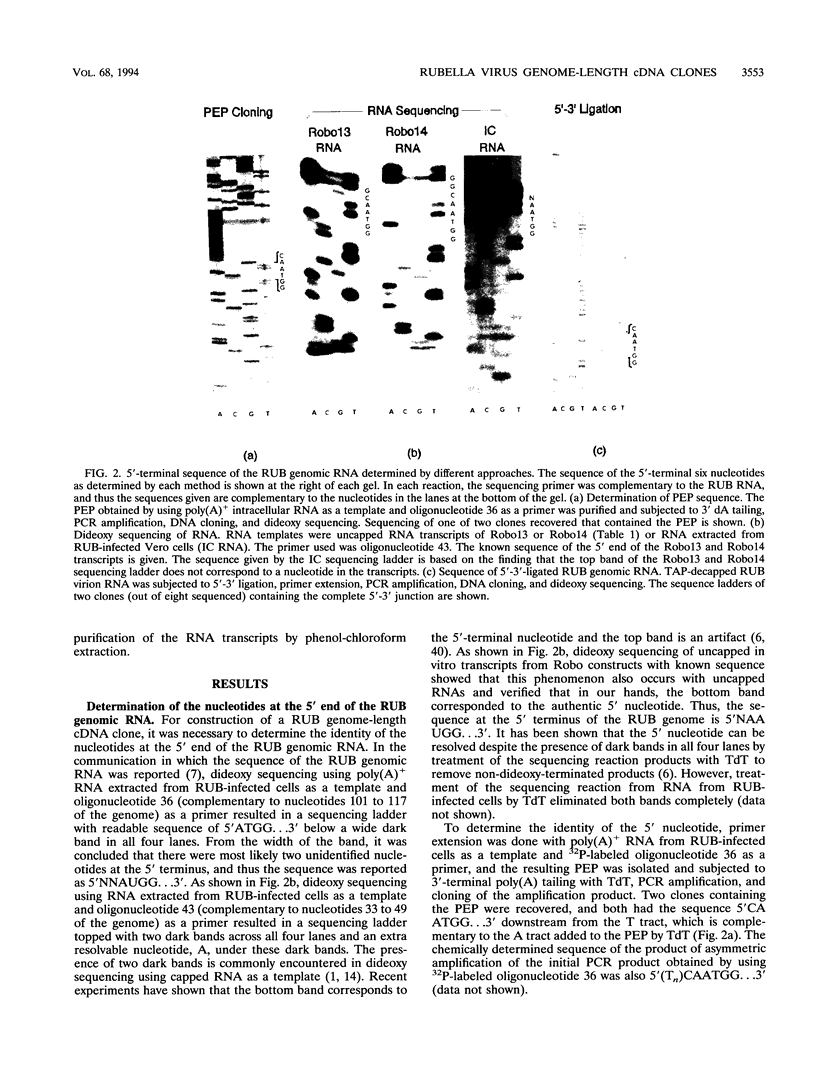
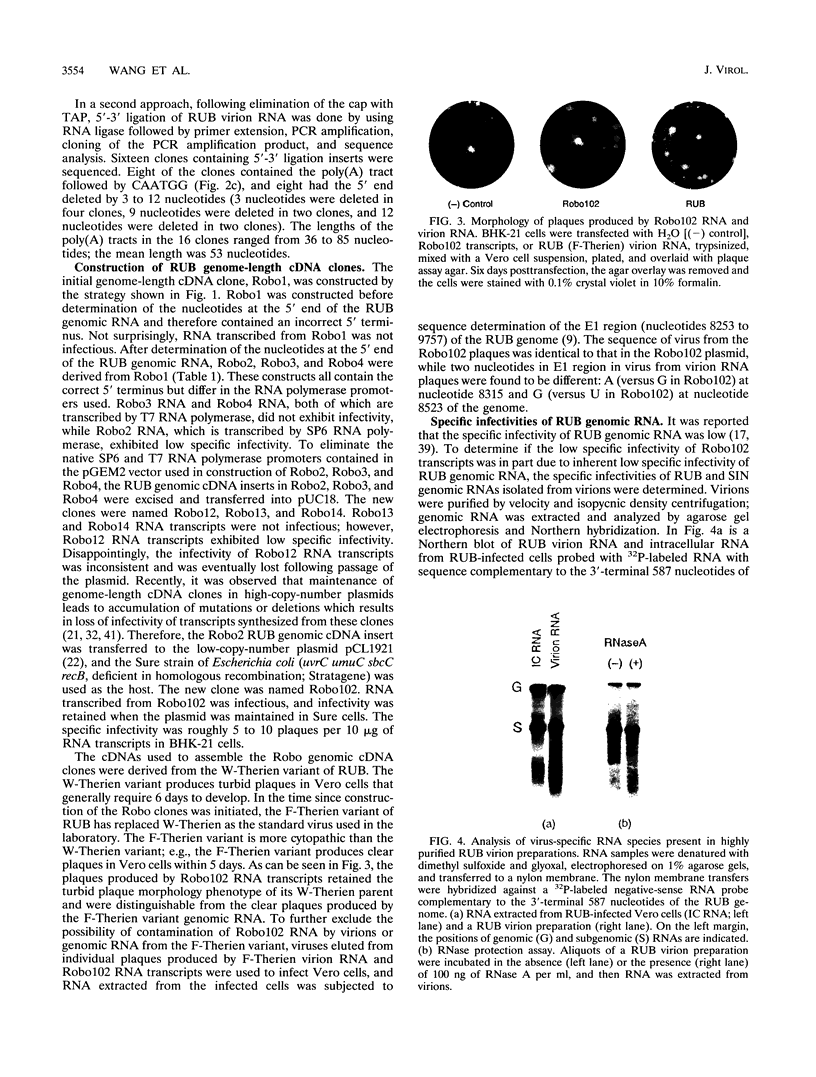
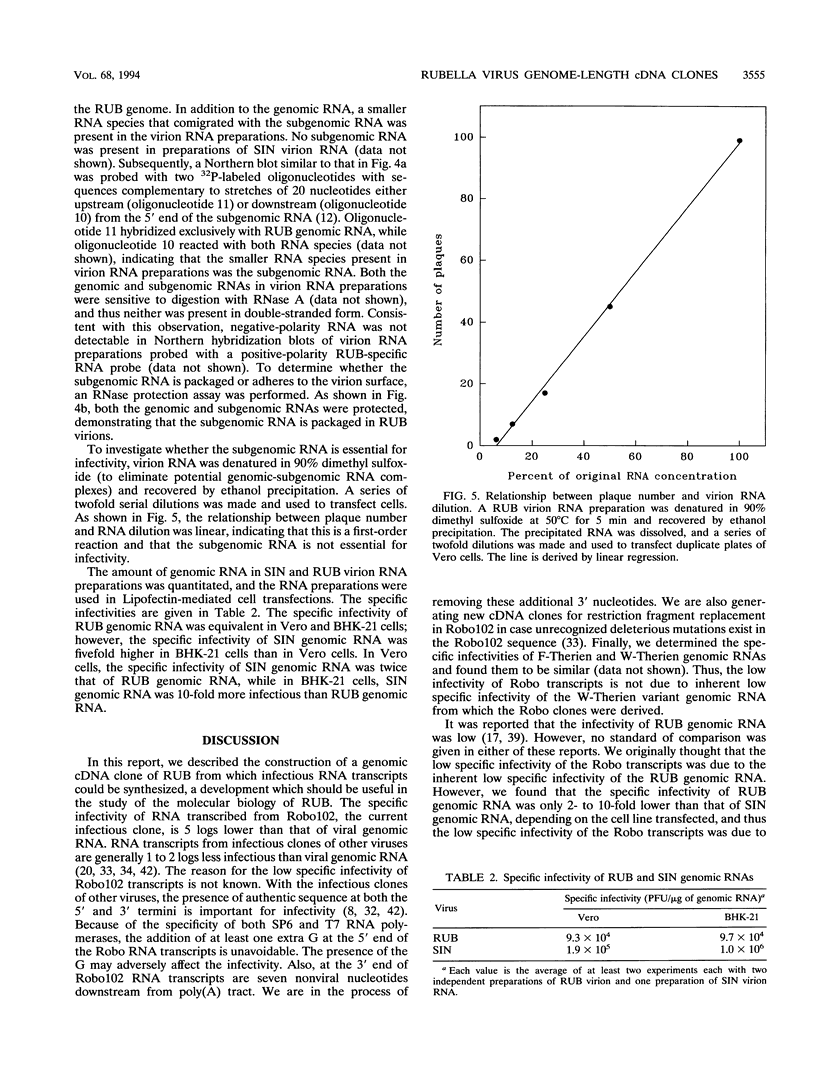
Plasmids containing a complete cDNA copy of the rubella virus (RUB) genomic RNA were constructed. Transfection into cell culture of genome-length RNA transcribed in vitro from one of these cDNA clones, Robo102, resulted in the production of virus which preserved the genetic and phenotypic characteristics of the parental virus from which the cDNA clone was derived. Prior to construction of the RUB genome-length cDNA clones, the 5'-terminal sequence of the RUB genomic RNA was determined to be 5'CAAUGG...3' following the cap structure. Analysis of the specific infectivity of RUB genomic RNA isolated from virions revealed that in Vero cells, the specific infectivity of RUB genomic RNA is roughly equivalent to that of Sindbis virus genomic RNA. In RUB virion RNA preparations, the subgenomic RNA was detected. It was demonstrated that subgenomic RNA was packaged into RUB virions; however, the presence of the subgenomic RNA was not essential for infectivity of the genomic RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund P., Sjöberg M., Garoff H., Atkins G. J., Sheahan B. J., Liljeström P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Biotechnology (N Y) 1993 Aug;11(8):916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- Bredenbeek P. J., Frolov I., Rice C. M., Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol. 1993 Nov;67(11):6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianetti L., Di Cristofaro A., Zappavigna V., Bottero L., Boccoli G., Testa U., Russo G., Boncinelli E., Peschle C. Molecular mechanisms underlying the expression of the human HOX-5.1 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4361–4368. doi: 10.1093/nar/18.15.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Willis L. V., Smith J. F., Johnston R. E. In vitro synthesis of infectious venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology. 1989 Jul;171(1):189–204. doi: 10.1016/0042-6822(89)90526-6. [DOI] [PubMed] [Google Scholar]
- DeBorde D. C., Naeve C. W., Herlocher M. L., Maassab H. F. Resolution of a common RNA sequencing ambiguity by terminal deoxynucleotidyl transferase. Anal Biochem. 1986 Sep;157(2):275–282. doi: 10.1016/0003-2697(86)90626-3. [DOI] [PubMed] [Google Scholar]
- Dominguez G., Wang C. Y., Frey T. K. Sequence of the genome RNA of rubella virus: evidence for genetic rearrangement during togavirus evolution. Virology. 1990 Jul;177(1):225–238. doi: 10.1016/0042-6822(90)90476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen R., Verver J., Wellink J., De Jong A., Goldbach R., van Kammen A. Improvements of the infectivity of in vitro transcripts from cloned cowpea mosaic virus cDNA: impact of terminal nucleotide sequences. Virology. 1989 Dec;173(2):447–455. doi: 10.1016/0042-6822(89)90557-6. [DOI] [PubMed] [Google Scholar]
- Frey T. K., Abernathy E. S. Identification of strain-specific nucleotide sequences in the RA 27/3 rubella virus vaccine. J Infect Dis. 1993 Oct;168(4):854–864. doi: 10.1093/infdis/168.4.854. [DOI] [PubMed] [Google Scholar]
- Frey T. K., Marr L. D., Hemphill M. L., Dominguez G. Molecular cloning and sequencing of the region of the rubella virus genome coding for glycoprotein E1. Virology. 1986 Oct 15;154(1):228–232. doi: 10.1016/0042-6822(86)90446-0. [DOI] [PubMed] [Google Scholar]
- Frey T. K., Marr L. D., Sanchez A., Simmons R. B. Identification of the 5' end of the rubella virus subgenomic RNA. Virology. 1989 Jan;168(1):191–194. doi: 10.1016/0042-6822(89)90422-4. [DOI] [PubMed] [Google Scholar]
- Frey T. K., Marr L. D. Sequence of the region coding for virion proteins C and E2 and the carboxy terminus of the nonstructural proteins of rubella virus: comparison with alphaviruses. Gene. 1988;62(1):85–99. doi: 10.1016/0378-1119(88)90582-3. [DOI] [PubMed] [Google Scholar]
- Frey T. K., Strauss J. H. Replication of Sindbis virus. VI. Poly(A) and poly(U) in virus-specific RNA species. Virology. 1978 May 15;86(2):494–506. doi: 10.1016/0042-6822(78)90088-0. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Complete sequences of the intergenic and mRNA start signals in the Sendai virus genome: homologies with the genome of vesicular stomatitis virus. Nucleic Acids Res. 1984 May 11;12(9):3829–3841. doi: 10.1093/nar/12.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill M. L., Forng R. Y., Abernathy E. S., Frey T. K. Time course of virus-specific macromolecular synthesis during rubella virus infection in Vero cells. Virology. 1988 Jan;162(1):65–75. doi: 10.1016/0042-6822(88)90395-9. [DOI] [PubMed] [Google Scholar]
- Ho-Terry L., Cohen A. Rubella virion polypeptides: characterization by polyacrylamide gel electrophoresis, isoelectric focusing and peptide mapping. Arch Virol. 1982;72(1-2):47–54. doi: 10.1007/BF01314449. [DOI] [PubMed] [Google Scholar]
- Hovi T., Vaheri A. Infectivity and some physicochemical characteristics of rubella virus ribonucleic acid. Virology. 1970 Sep;42(1):1–8. doi: 10.1016/0042-6822(70)90232-1. [DOI] [PubMed] [Google Scholar]
- Kuhn R. J., Niesters H. G., Hong Z., Strauss J. H. Infectious RNA transcripts from Ross River virus cDNA clones and the construction and characterization of defined chimeras with Sindbis virus. Virology. 1991 Jun;182(2):430–441. doi: 10.1016/0042-6822(91)90584-x. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Zhao B. T., Hori H., Bray M. Infectious RNA transcribed from stably cloned full-length cDNA of dengue type 4 virus. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5139–5143. doi: 10.1073/pnas.88.12.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., Lusa S., Huylebroeck D., Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991 Aug;65(8):4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl C. W., Heinz F. X., Puchhammer-Stöckl E., Kunz C. Sequencing the termini of capped viral RNA by 5'-3' ligation and PCR. Biotechniques. 1991 Apr;10(4):484–486. [PubMed] [Google Scholar]
- Marr L. D., Sanchez A., Frey T. K. Efficient in vitro translation and processing of the rubella virus structural proteins in the presence of microsomes. Virology. 1991 Jan;180(1):400–405. doi: 10.1016/0042-6822(91)90046-e. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Norval M. Mechanism of persistence of rubella virus in LLC-MK2 cells. J Gen Virol. 1979 May;43(2):289–298. doi: 10.1099/0022-1317-43-2-289. [DOI] [PubMed] [Google Scholar]
- Oker-Blom C., Kalkkinen N., Käriäinen L., Pettersson R. F. Rubella virus contains one capsid protein and three envelope glycoproteins, E1, E2a, and E2b. J Virol. 1983 Jun;46(3):964–973. doi: 10.1128/jvi.46.3.964-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oker-Blom C., Ulmanen I., Käriäinen L., Pettersson R. F. Rubella virus 40S genome RNA specifies a 24S subgenomic mRNA that codes for a precursor to structural proteins. J Virol. 1984 Feb;49(2):403–408. doi: 10.1128/jvi.49.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Strauss E. G., Strauss J. H. The 5'-terminal sequences of the genomic RNAs of several alphaviruses. J Mol Biol. 1983 Jul 25;168(1):1–15. doi: 10.1016/s0022-2836(83)80319-2. [DOI] [PubMed] [Google Scholar]
- Quillet L., Guilley H., Jonard G., Richards K. In vitro synthesis of biologically active beet necrotic yellow vein virus RNA. Virology. 1989 Sep;172(1):293–301. doi: 10.1016/0042-6822(89)90131-1. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Grakoui A., Galler R., Chambers T. J. Transcription of infectious yellow fever RNA from full-length cDNA templates produced by in vitro ligation. New Biol. 1989 Dec;1(3):285–296. [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Stein S., Ohara Y., Fu J. L., Semler B. L. Infectious cDNA clones of the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1989 Dec;63(12):5492–5496. doi: 10.1128/jvi.63.12.5492-5496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümenapf T., Strauss E. G., Strauss J. H. Subgenomic mRNA of Aura alphavirus is packaged into virions. J Virol. 1994 Jan;68(1):56–62. doi: 10.1128/jvi.68.1.56-62.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A., Frey T. K. Vaccinia-vectored expression of the rubella virus structural proteins and characterization of the E1 and E2 glycosidic linkages. Virology. 1991 Aug;183(2):636–646. doi: 10.1016/0042-6822(91)90993-l. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Sokol F. Nucleic acid of rubella virus and its replication in hamster kidney cells. J Virol. 1970 Apr;5(4):478–489. doi: 10.1128/jvi.5.4.478-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y., Niklasson B., Dalrymple J. M., Strauss E. G., Strauss J. H. Structure of the Ockelbo virus genome and its relationship to other Sindbis viruses. Virology. 1991 Jun;182(2):753–764. doi: 10.1016/0042-6822(91)90616-j. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi H., Hoke C. H., Trent D. W. Infectious Japanese encephalitis virus RNA can be synthesized from in vitro-ligated cDNA templates. J Virol. 1992 Sep;66(9):5425–5431. doi: 10.1128/jvi.66.9.5425-5431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P., Verver J., van Wezenbeek P., van Kammen A., Goldbach R. Study of the genetic organisation of a plant viral RNA genome by in vitro expression of a full-length DNA copy. EMBO J. 1984 Dec 20;3(13):3049–3053. doi: 10.1002/j.1460-2075.1984.tb02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. Immunochemical identification of rubella virus hemagglutinin. Virology. 1983 Apr 15;126(1):194–203. doi: 10.1016/0042-6822(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Weiss B., Nitschko H., Ghattas I., Wright R., Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J Virol. 1989 Dec;63(12):5310–5318. doi: 10.1128/jvi.63.12.5310-5318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. T., Robinson W. S., Merigan T. C. Synthesis of viral-specific ribonucleic Acid in rubella virus-infected cells. J Virol. 1969 Dec;4(6):901–903. doi: 10.1128/jvi.4.6.901-903.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C., Levis R., Shen P., Schlesinger S., Rice C. M., Huang H. V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989 Mar 3;243(4895):1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bradley J., Wimmer E., Studier F. W., Dunn J. J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]