Abstract
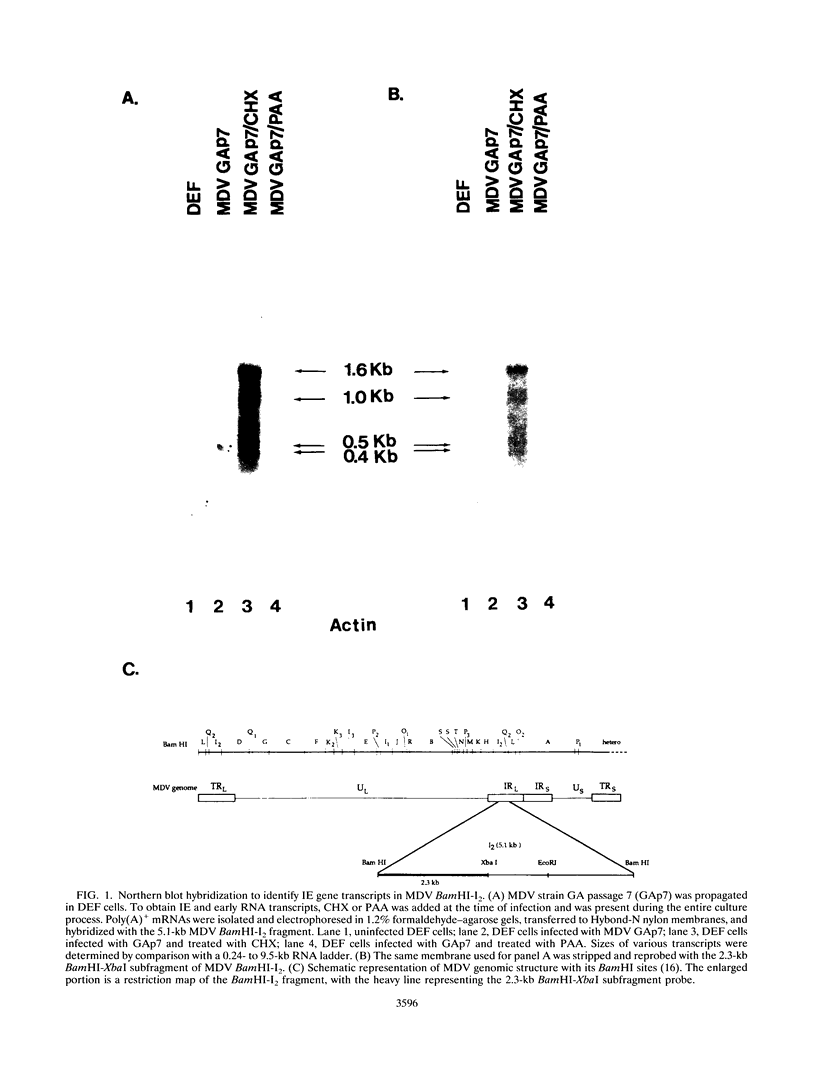
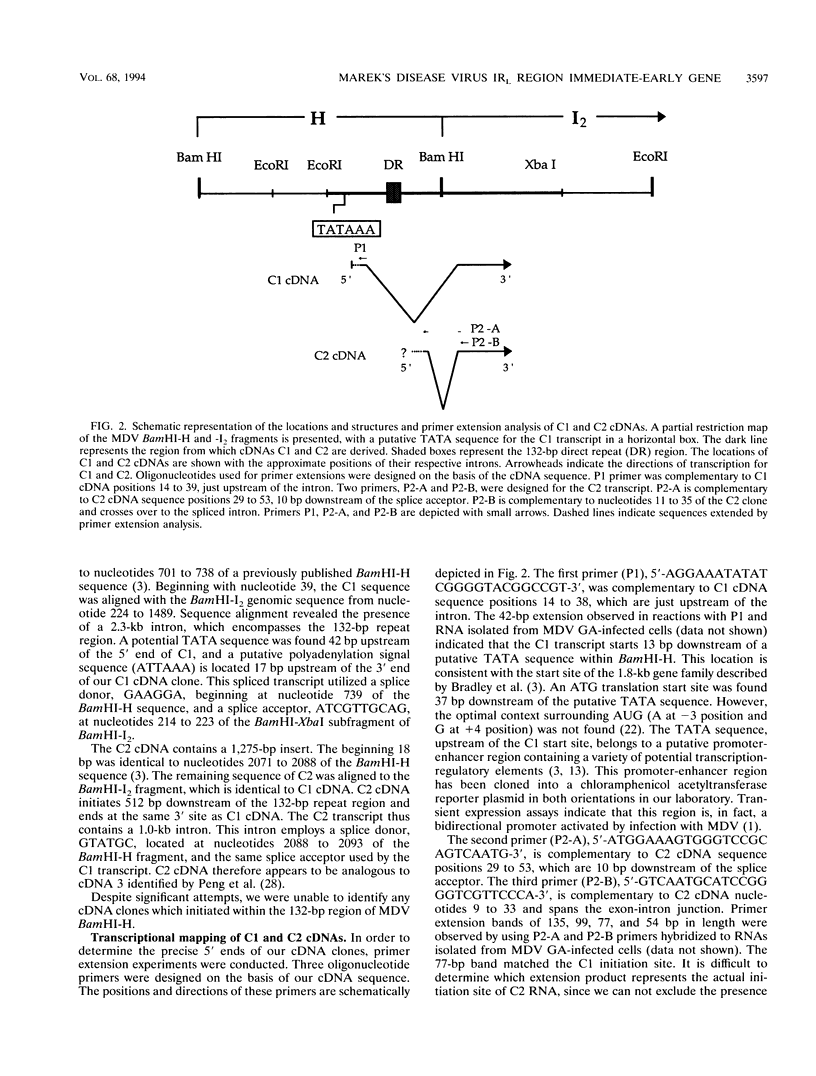
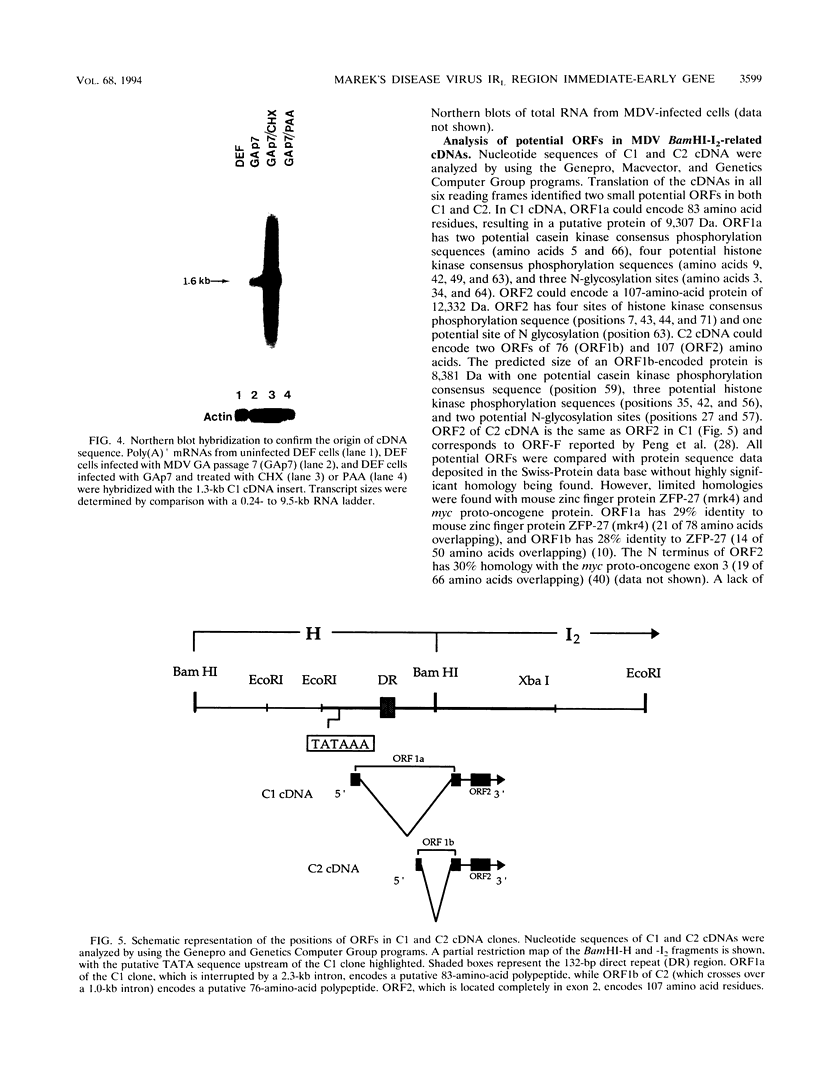
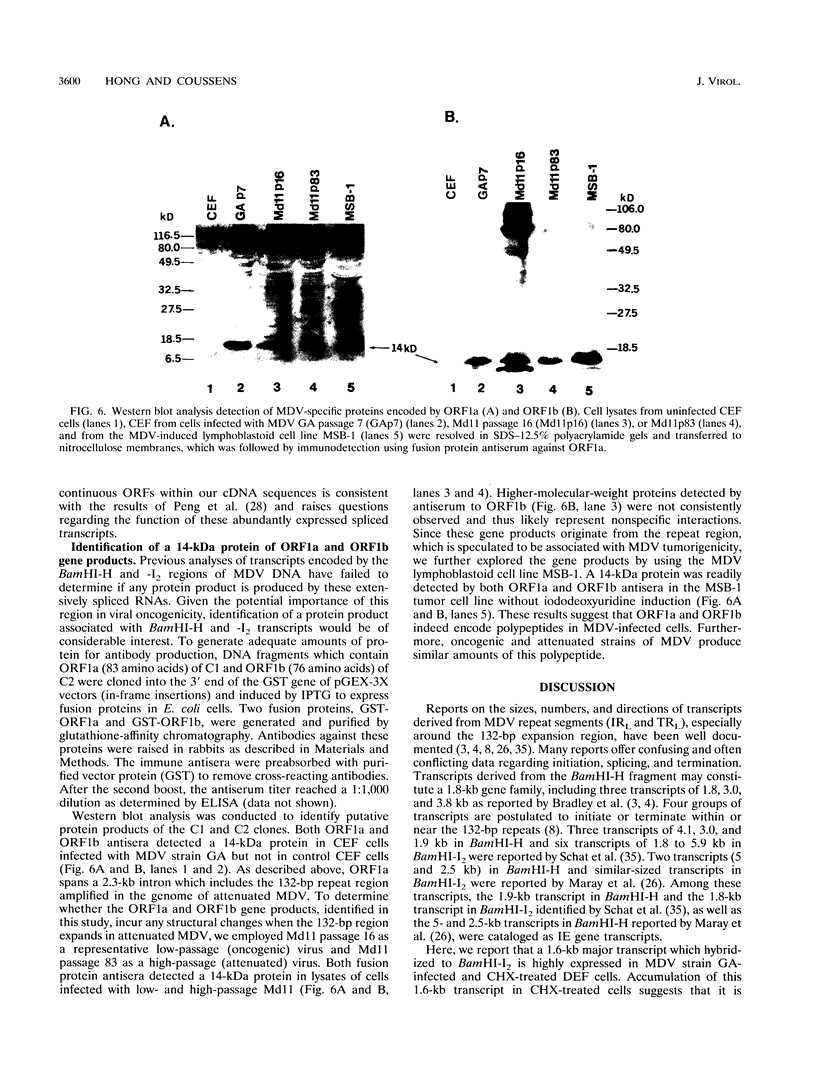
Marek's disease virus (MDV) is an oncogenic avian herpesvirus whose genomic structure is similar to those of herpes simplex virus and varicella-zoster virus. Repeat regions of the MDV genome have been intensively investigated because of a potential relationship to MDV oncogenicity and abundant expression of immediate-early transcripts. In this study, a 1.6-kb immediate-early transcript was localized to the BamHI-I2 region by Northern (RNA) hybridization analysis. With cDNA cloning and sequencing, two cDNAs of 1.4 kb (C1) and 1.35 kb (C2) were identified. Both cDNAs are derived from spliced mRNAs spanning the BamHI-H and -I2 fragments. C1 and C2 use the same splice acceptors and 3' ends, but they differ at their 5' ends and utilize different splice donors. The upstream promoter-enhancer region of C1 cDNA has been defined as a bidirectional regulatory region shared by the MDV pp38 gene. Sequencing analysis shows two small open reading frames (ORFs) within each cDNA (ORF1a and ORF2 in C1, ORF1b and ORF2 in C2). Potential ORFs of the sequence have no significant homology with any known protein in the Swiss-Protein data base. DNA fragments encoding ORF1a and ORF1b were cloned into pGEX-3X vectors to produce glutathione S-transferase fusion proteins and induce antisera. In Western blot (immunoblot) analysis of MDV-infected-cell lysates, a 14-kDa polypeptide was identified by antisera against both ORF1a and ORF1b. This 14-kDa protein is expressed in cells which are lytically infected with MDV strains GA, Md11 passage 14 (oncogenic), and Md11 passage 83 (attenuated), as well as in the latently MDV-infected and transformed MSB-1 cell line.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Bradley G., Hayashi M., Lancz G., Tanaka A., Nonoyama M. Structure of the Marek's disease virus BamHI-H gene family: genes of putative importance for tumor induction. J Virol. 1989 Jun;63(6):2534–2542. doi: 10.1128/jvi.63.6.2534-2542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Lancz G., Tanaka A., Nonoyama M. Loss of Marek's disease virus tumorigenicity is associated with truncation of RNAs transcribed within BamHI-H. J Virol. 1989 Oct;63(10):4129–4135. doi: 10.1128/jvi.63.10.4129-4135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek B. W. Marek's disease--a model for herpesvirus oncology. Crit Rev Microbiol. 1986;12(4):293–320. doi: 10.3109/10408418509104432. [DOI] [PubMed] [Google Scholar]
- Cebrian J., Kaschka-Dierich C., Berthelot N., Sheldrick P. Inverted repeat nucleotide sequences in the genomes of Marek disease virus and the herpesvirus of the turkey. Proc Natl Acad Sci U S A. 1982 Jan;79(2):555–558. doi: 10.1073/pnas.79.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. B., Sondermeijer P. J., Velicer L. F. Identification of a unique Marek's disease virus gene which encodes a 38-kilodalton phosphoprotein and is expressed in both lytically infected cells and latently infected lymphoblastoid tumor cells. J Virol. 1992 Jan;66(1):85–94. doi: 10.1128/jvi.66.1.85-94.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. B., Velicer L. F. Multiple bidirectional initiations and terminations of transcription in the Marek's disease virus long repeat regions. J Virol. 1991 May;65(5):2445–2451. doi: 10.1128/jvi.65.5.2445-2451.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chowdhury K., Rohdewohld H., Gruss P. Specific and ubiquitous expression of different Zn finger protein genes in the mouse. Nucleic Acids Res. 1988 Nov 11;16(21):9995–10011. doi: 10.1093/nar/16.21.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill A. E., Biggs P. M. Agent of Marek's disease in tissue culture. Nature. 1967 Jul 29;215(5100):528–530. doi: 10.1038/215528a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cui Z. Z., Lee L. F., Liu J. L., Kung H. J. Structural analysis and transcriptional mapping of the Marek's disease virus gene encoding pp38, an antigen associated with transformed cells. J Virol. 1991 Dec;65(12):6509–6515. doi: 10.1128/jvi.65.12.6509-6515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka A., Schierman L. W., Witter R. L., Nonoyama M. The structure of Marek disease virus DNA: the presence of unique expansion in nonpathogenic viral DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(3):751–754. doi: 10.1073/pnas.82.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaubiger C., Nazerian K., Velicer L. F. Marek's disease herpesviruses. IV. Molecular characterization of Marek's disease herpesvirus A antigen. J Virol. 1983 Mar;45(3):1228–1234. doi: 10.1128/jvi.45.3.1228-1234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. N., Kopachik W., Band R. N. A simple, efficient method to create a cDNA library. Biotechniques. 1992 Dec;13(6):862–864. [PubMed] [Google Scholar]
- Iwata A., Ueda S., Ishihama A., Hirai K. Sequence determination of cDNA clones of transcripts from the tumor-associated region of the Marek's disease virus genome. Virology. 1992 Apr;187(2):805–808. doi: 10.1016/0042-6822(92)90483-6. [DOI] [PubMed] [Google Scholar]
- Jones D., Lee L., Liu J. L., Kung H. J., Tillotson J. K. Marek disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4042–4046. doi: 10.1073/pnas.89.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M., Hayashi M., Furuichi T., Nonoyama M., Isogai E., Namioka S. The inhibitory effects of oligonucleotides, complementary to Marek's disease virus mRNA transcribed from the BamHI-H region, on the proliferation of transformed lymphoblastoid cells, MDCC-MSB1. J Gen Virol. 1991 May;72(Pt 5):1105–1111. doi: 10.1099/0022-1317-72-5-1105. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Lau R., Packham G., Farrell P. J. Differential splicing of Epstein-Barr virus immediate-early RNA. J Virol. 1992 Oct;66(10):6233–6236. doi: 10.1128/jvi.66.10.6233-6236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Coen D. M., Bogard C. L., Hicks K. A., Yager D. R., Knipe D. M., Tyler K. L., Schaffer P. A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989 Feb;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maotani K., Kanamori A., Ikuta K., Ueda S., Kato S., Hirai K. Amplification of a tandem direct repeat within inverted repeats of Marek's disease virus DNA during serial in vitro passage. J Virol. 1986 May;58(2):657–660. doi: 10.1128/jvi.58.2.657-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maray T., Malkinson M., Becker Y. RNA transcripts of Marek's disease virus (MDV) serotype-1 in infected and transformed cells. Virus Genes. 1988 Oct;2(1):49–68. doi: 10.1007/BF00569736. [DOI] [PubMed] [Google Scholar]
- Nazerian K., Lee L. F. Selective inhibition by phosphonoacetic acid of MDV DNA replication in a lymphoblastoid cell line. Virology. 1976 Oct 1;74(1):188–193. doi: 10.1016/0042-6822(76)90140-9. [DOI] [PubMed] [Google Scholar]
- Peng F., Bradley G., Tanaka A., Lancz G., Nonoyama M. Isolation and characterization of cDNAs from BamHI-H gene family RNAs associated with the tumorigenicity of Marek's disease virus. J Virol. 1992 Dec;66(12):7389–7396. doi: 10.1128/jvi.66.12.7389-7396.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Carmichael L. E., Deinhardt F., de-The G., Nahmias A. J., Plowright W., Rapp F., Sheldrick P., Takahashi M., Wolf K. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology. 1981;16(4):201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat K. A., Buckmaster A., Ross L. J. Partial transcription map of Marek's disease herpesvirus in lytically infected cells and lymphoblastoid cell lines. Int J Cancer. 1989 Jul 15;44(1):101–109. doi: 10.1002/ijc.2910440119. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Witter R. L. Genomic expansion of Marek's disease virus DNA is associated with serial in vitro passage. J Virol. 1985 Jun;54(3):690–696. doi: 10.1128/jvi.54.3.690-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beneden R. J., Watson D. K., Chen T. T., Lautenberger J. A., Papas T. S. Cellular myc (c-myc) in fish (rainbow trout): its relationship to other vertebrate myc genes and to the transforming genes of the MC29 family of viruses. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3698–3702. doi: 10.1073/pnas.83.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R., Southwick R. A., Pulaski J. T., Tieber V. L., Hong Y., Coussens P. M. Molecular analysis of the glycoprotein C-negative phenotype of attenuated Marek's disease virus. Virology. 1994 Mar;199(2):393–402. doi: 10.1006/viro.1994.1137. [DOI] [PubMed] [Google Scholar]
- Wong S. W., Schaffer P. A. Elements in the transcriptional regulatory region flanking herpes simplex virus type 1 oriS stimulate origin function. J Virol. 1991 May;65(5):2601–2611. doi: 10.1128/jvi.65.5.2601-2611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen V. L. Characterization of a bovine herpesvirus 4 immediate-early RNA encoding a homolog of the Epstein-Barr virus R transactivator. J Virol. 1993 Feb;67(2):773–784. doi: 10.1128/jvi.67.2.773-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]