Abstract
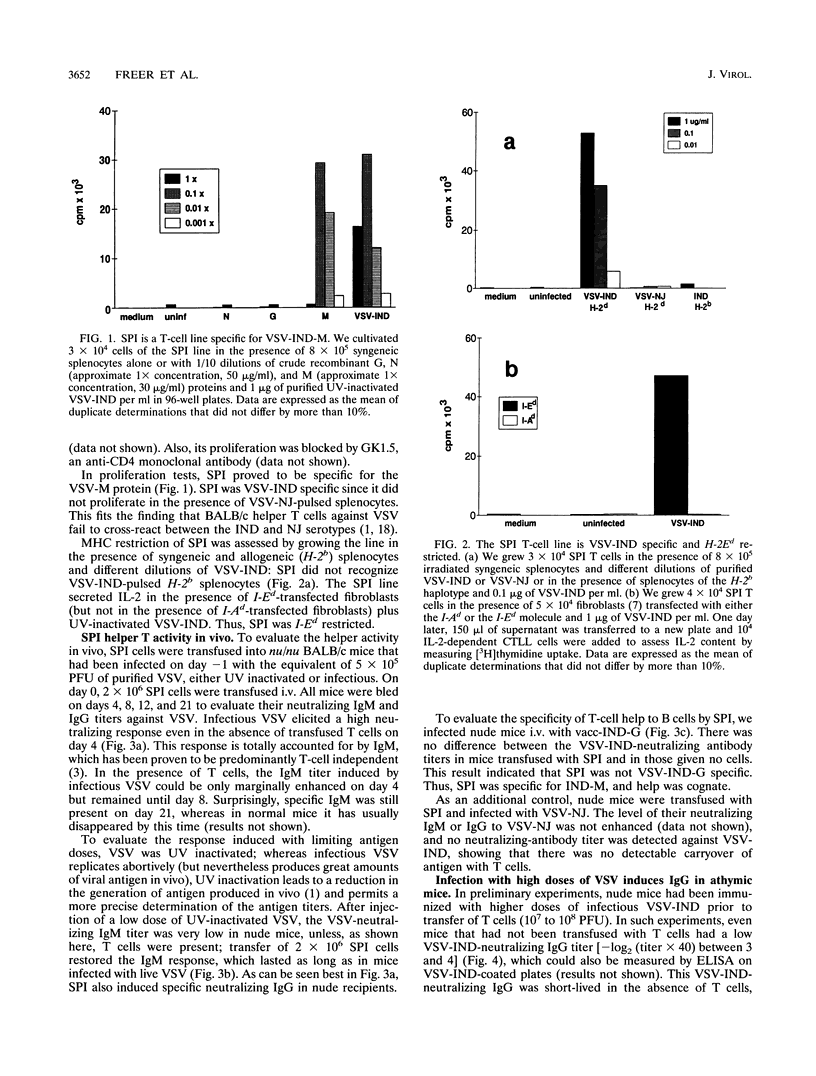
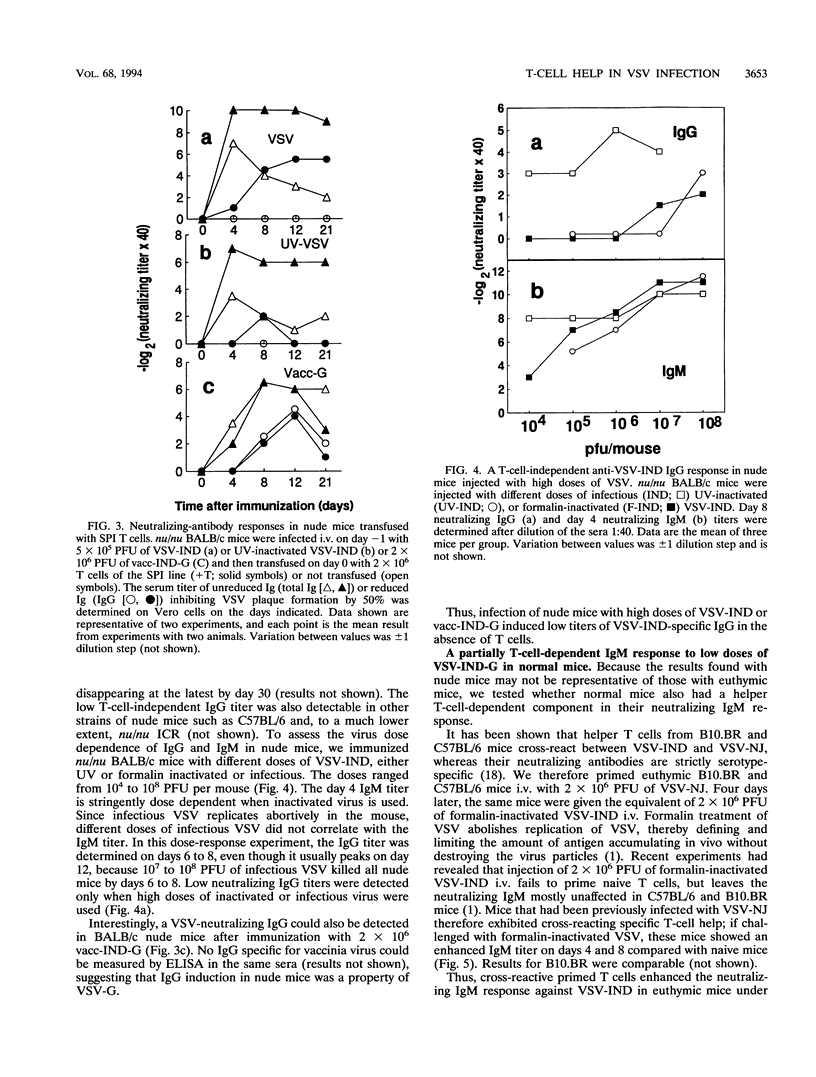
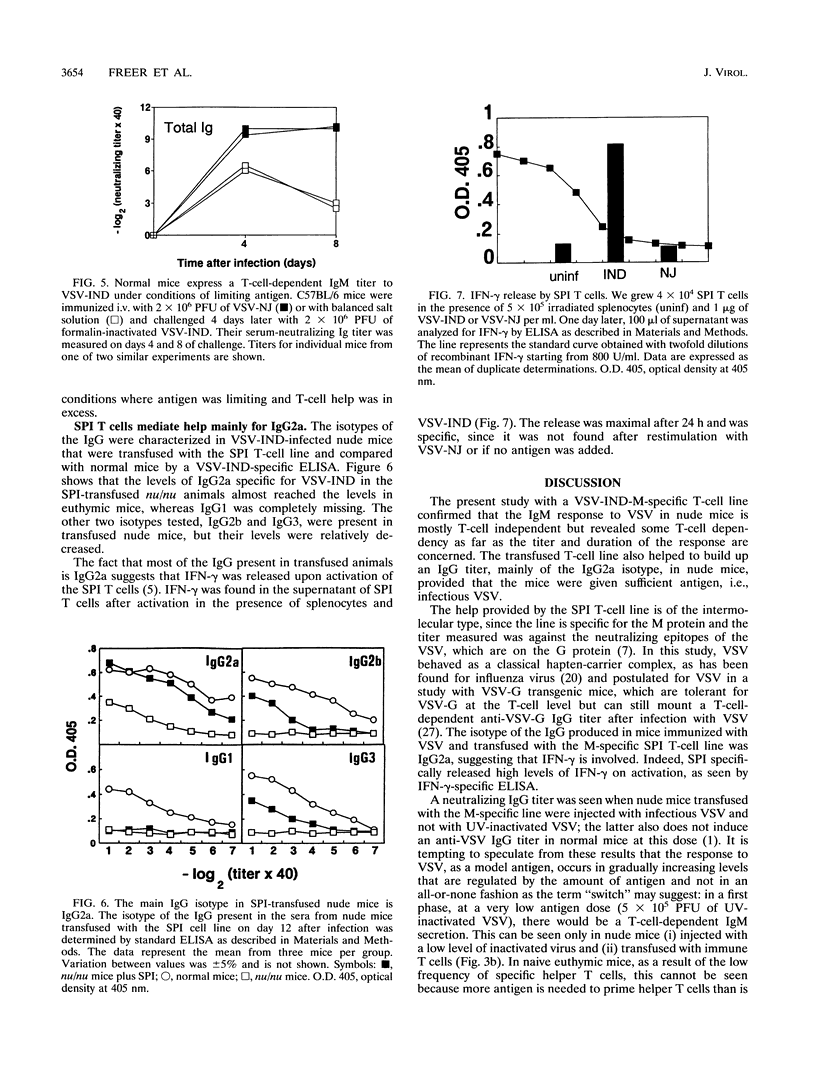
The neutralizing immunoglobulin M (IgM) response to vesicular stomatitis virus (VSV) has been shown to be largely T-cell independent in several T-cell-deficient models of mice. By using different antigen froms of VSV, VSV antigen doses could be graded in vivo (infectious > > UV inactivated > formalin inactivated). The present study reveals a T-cell-dependent component of the neutralizing IgM response in nude mice given intravenous injections of low doses of noninfectious UV-inactivated VSV serotype Indiana (VSV-IND) only if the mice are transfused with VSV-IND-specific helper T cells. Instead, nude mice immunized with infectious VSV, which leads to greater antigen doses in vivo, were able to mount an IgM response in the absence of T cells. These results indicate that the IgM response to low doses of VSV-IND glycoprotein (G) is T-cell dependent. Nude mice immunized with infectious VSV also made a variable but low VSV-IND-neutralizing IgG response. A VSV-IND matrix (M)-specific helper T-cell line rendered this response more consistent, much higher, and longer lasting. Thus (i) VSV-G induces a mostly T-cell-independent but partially T-cell-dependent IgM (the latter can be visualized best at low doses of antigen) and (ii) the antibody response to VSV in nude mice proceeds through steps, i.e., IgM and IgG, that are dose dependent. The results suggest that the predominant role of helper T cells may be to expand and maintain the individual steps of differentiating B cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M. F., Kündig T. M., Kalberer C. P., Hengartner H., Zinkernagel R. M. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J Virol. 1993 Jul;67(7):3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. J., McLeod D. A., Kang C. Y., Bishop D. H. Glycosylation is not required for the fusion activity of the G protein of vesicular stomatitis virus in insect cells. Virology. 1989 Apr;169(2):323–331. doi: 10.1016/0042-6822(89)90157-8. [DOI] [PubMed] [Google Scholar]
- Charan S., Zinkernagel R. M. Antibody mediated suppression of secondary IgM response in nude mice against vesicular stomatitis virus. J Immunol. 1986 Apr 15;136(8):3057–3061. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Gao X. M., Liew F. Y., Tite J. P. Identification and characterization of T helper epitopes in the nucleoprotein of influenza A virus. J Immunol. 1989 Nov 1;143(9):3007–3014. [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler R. I., Norcross M. A., Germain R. N. Qualitative and quantitative studies of antigen-presenting cell function by using I-A-expressing L cells. J Immunol. 1985 Nov;135(5):2914–2922. [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Lukacher A. E., Morrison L. A., Braciale V. L., Malissen B., Braciale T. J. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985 Jul 1;162(1):171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986 Dec 12;234(4782):1398–1401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Braciale V. L., Braciale T. J. Antigen form influences induction and frequency of influenza-specific class I and class II MHC-restricted cytolytic T lymphocytes. J Immunol. 1988 Jul 15;141(2):363–368. [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Möller G. One non-specific signal triggers b lymphocytes. Transplant Rev. 1975;23:126–137. [PubMed] [Google Scholar]
- Osler A. G. Immunology of reaginic allergy: in vitro studies. Clin Exp Immunol. 1970 Jan;6(1):13–23. [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roost H. P., Charan S., Zinkernagel R. M. Analysis of the kinetics of antiviral memory T help in vivo: characterization of short-lived cross-reactive T help. Eur J Immunol. 1990 Dec;20(12):2547–2554. doi: 10.1002/eji.1830201204. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Cross-reactive cytotoxic T cells to serologically distinct vesicular stomatitis virus. J Immunol. 1980 May;124(5):2301–2308. [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P. A., Palladino G., Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J Immunol. 1992 Jan 1;148(1):212–217. [PubMed] [Google Scholar]
- Speiser D. E., Stübi U., Zinkernagel R. M. Extrathymic positive selection of alpha beta T-cell precursors in nude mice. Nature. 1992 Jan 9;355(6356):170–172. doi: 10.1038/355170a0. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Hackett C. J., Askonas B. A. Evidence for two T-helper populations with distinct specificity in the humoral response to influenza A viruses. Immunology. 1982 Nov;47(3):429–436. [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Berton M. T., Burger C., Kepron M., Lee W. T., Yin X. M. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Cooper S., Chambers J., Lazzarini R. A., Hengartner H., Arnheiter H. Virus-induced autoantibody response to a transgenic viral antigen. Nature. 1990 May 3;345(6270):68–71. doi: 10.1038/345068a0. [DOI] [PubMed] [Google Scholar]


