Abstract
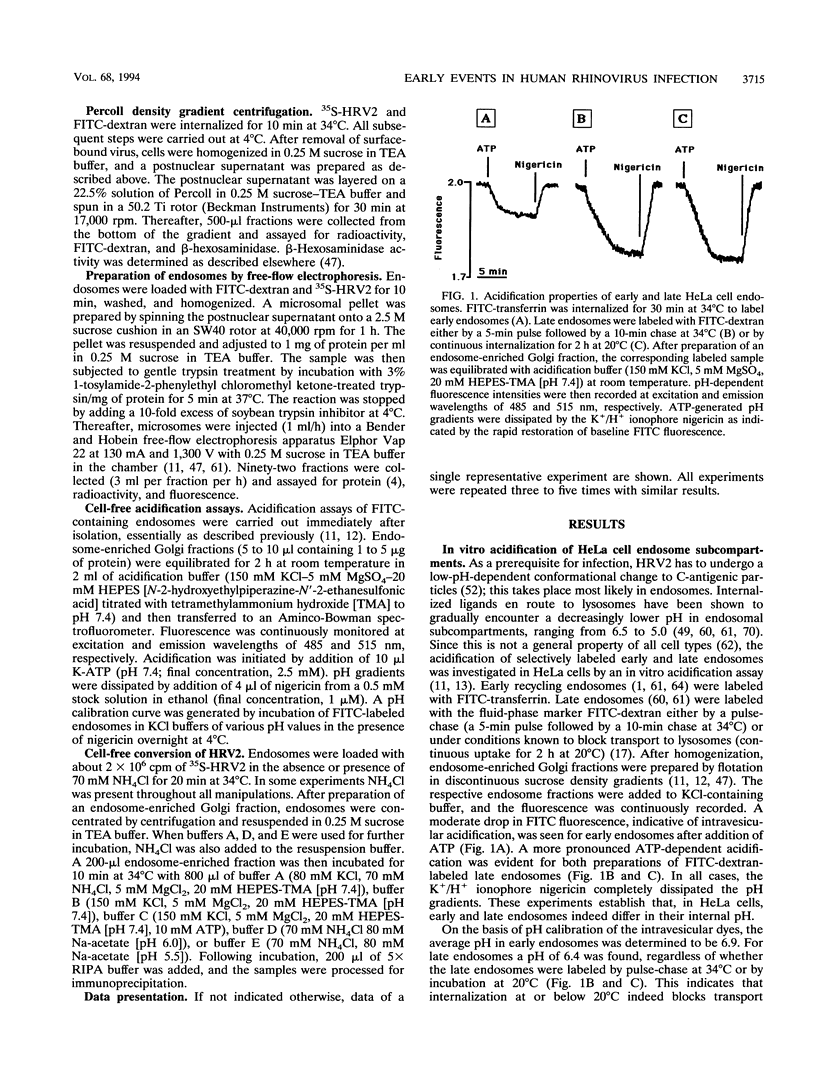
The internalization pathway and mechanism of uncoating of human rhinovirus serotype 2 (HRV2), a minor-group human rhinovirus, were investigated. Kinetic analysis revealed a late endosomal compartment as the site of capsid modification from D to C antigenicity. The conformational change as well as the infection was prevented by the specific V-ATPase inhibitor bafilomycin A1. A requirement for ATP was also demonstrated with purified endosomes in vitro. Capsid modifications occurred at a pH of 5.5 regardless of whether the virus was entrapped in isolated endosomes or free in solution. These findings suggest that the receptor is not directly involved in the structural modification of HRV2. Viral particles found in purified endosomes of infected cells were mostly devoid of RNA. This supports the hypothesis that uncoating of HRV2 occurs in intact endosomes rather than by a mechanism involving endosomal disruption with subsequent release of the RNA into the cytoplasm.
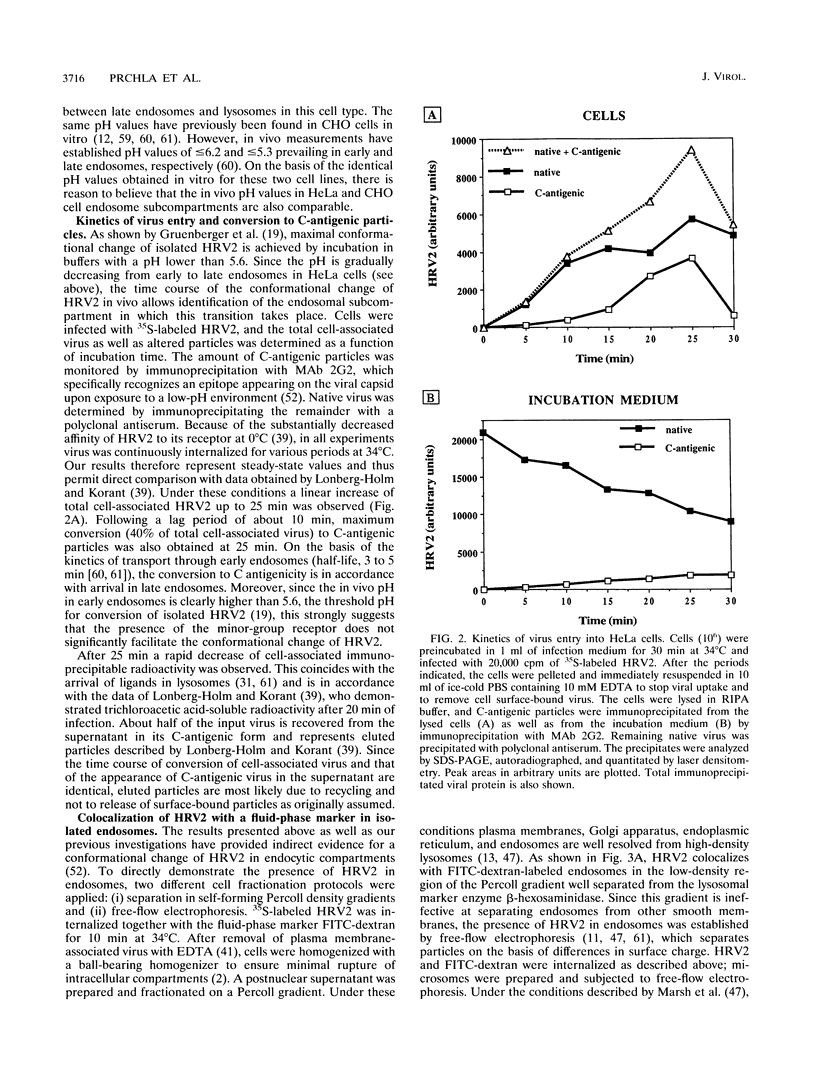
Full text
PDF

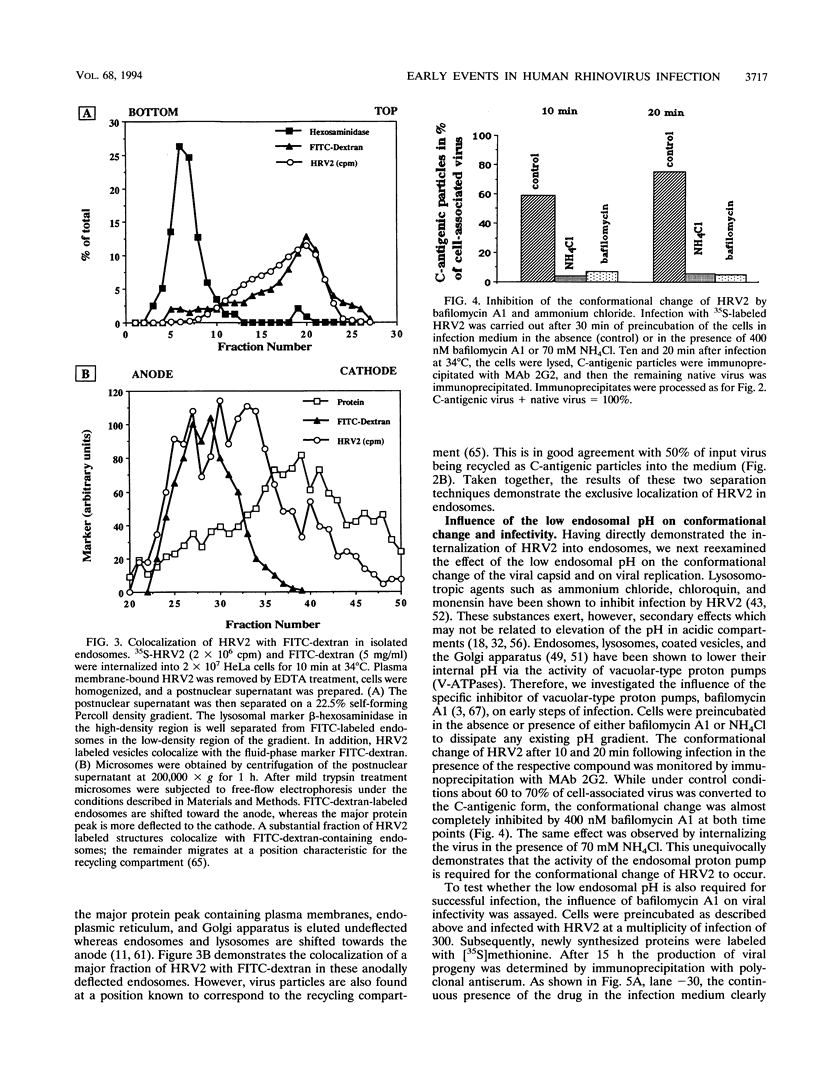
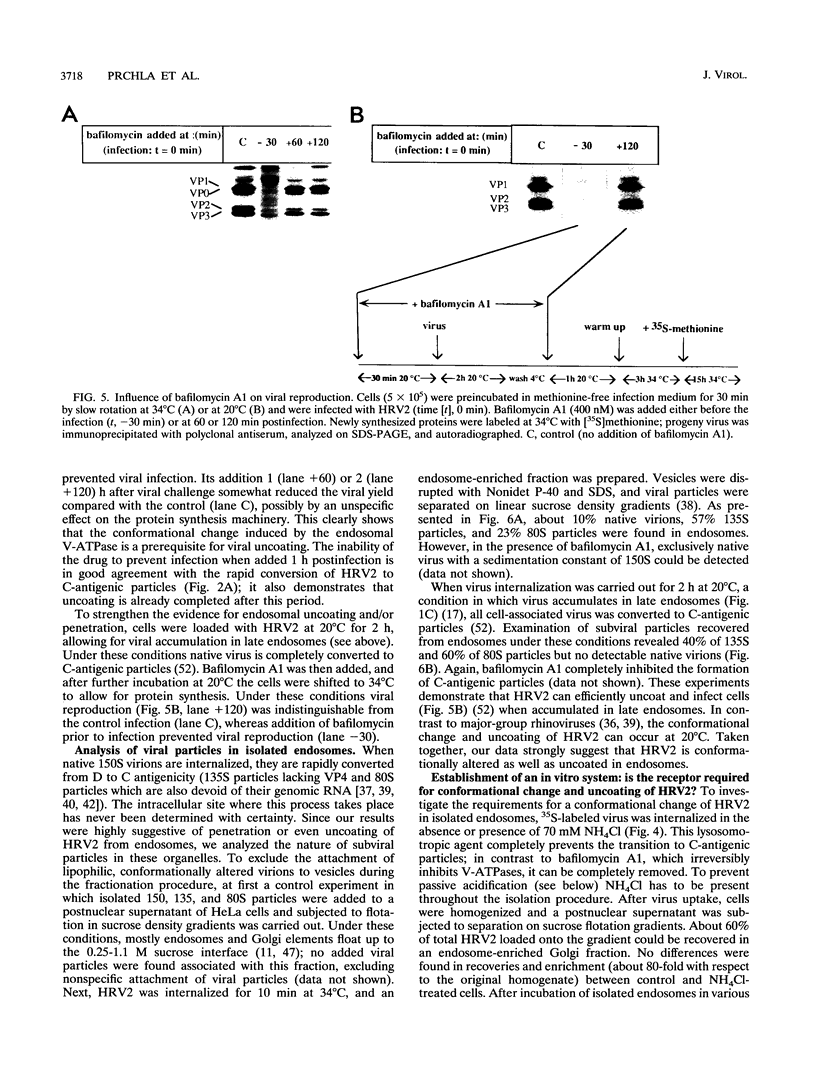



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
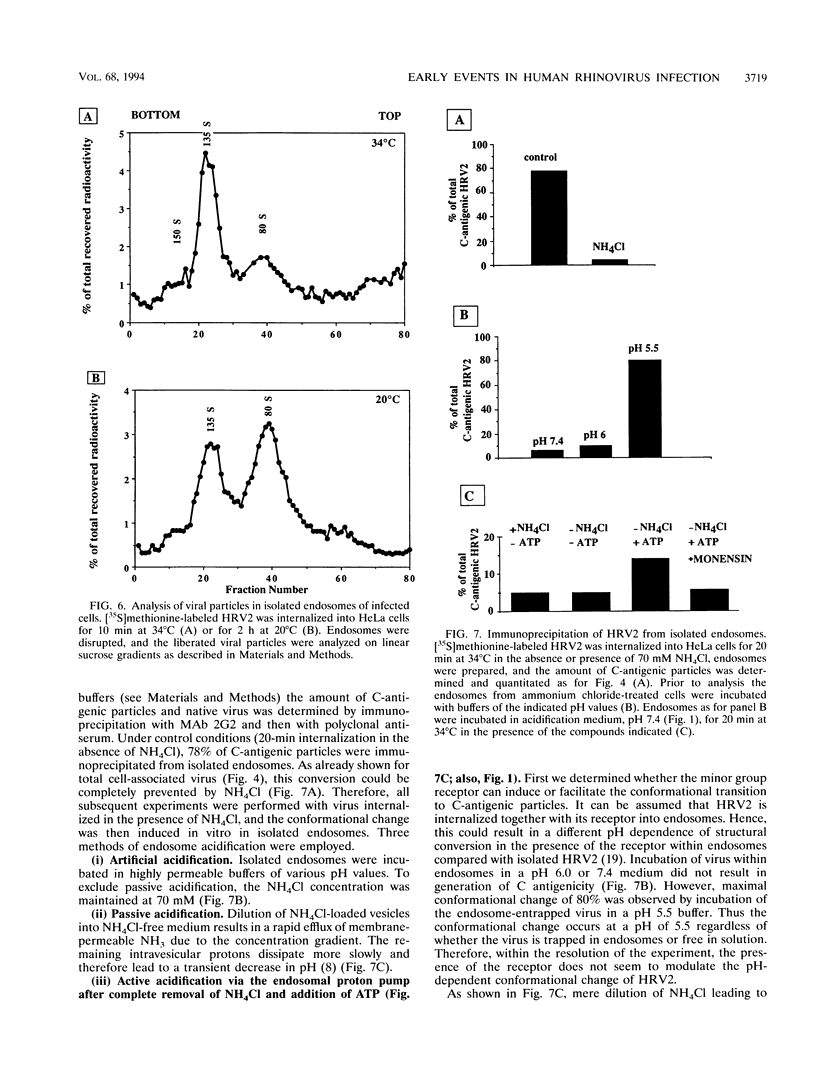
- Ajioka R. S., Kaplan J. Characterization of endocytic compartments using the horseradish peroxidase-diaminobenzidine density shift technique. J Cell Biol. 1987 Jan;104(1):77–85. doi: 10.1083/jcb.104.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Rothman J. E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985 Jul;240(1):413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sena J., Mandel B. Studies on the in vitro uncoating of poliovirus. I. Characterization of the modifying factor and the modifying reaction. Virology. 1976 Apr;70(2):470–483. doi: 10.1016/0042-6822(76)90288-9. [DOI] [PubMed] [Google Scholar]
- Everaert L., Vrijsen R., Boeyé A. Eclipse products of poliovirus after cold-synchronized infection of HeLa cells. Virology. 1989 Jul;171(1):76–82. doi: 10.1016/0042-6822(89)90512-6. [DOI] [PubMed] [Google Scholar]
- Felberbauer F. X., Graf J. Fiber-optic measurement of intracellular pH in intact rat liver using pH-sensitive dyes. Pflugers Arch. 1991 Sep;419(2):215–217. doi: 10.1007/BF00373011. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Mâle P., Mellman I. Acidification and ion permeabilities of highly purified rat liver endosomes. J Biol Chem. 1989 Feb 5;264(4):2212–2220. [PubMed] [Google Scholar]
- Fuchs R., Schmid S., Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci U S A. 1989 Jan;86(2):539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Willetts M., Webster P., Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993 Nov 5;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Green S. A., Kelly R. B. Endocytic membrane traffic to the Golgi apparatus in a regulated secretory cell line. J Biol Chem. 1990 Dec 5;265(34):21269–21278. [PubMed] [Google Scholar]
- Greve J. M., Forte C. P., Marlor C. W., Meyer A. M., Hoover-Litty H., Wunderlich D., McClelland A. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J Virol. 1991 Nov;65(11):6015–6023. doi: 10.1128/jvi.65.11.6015-6023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Hoflack B., Simons K., Mellman I., Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988 Feb 12;52(3):329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Gromeier M., Wetz K. Kinetics of poliovirus uncoating in HeLa cells in a nonacidic environment. J Virol. 1990 Aug;64(8):3590–3597. doi: 10.1128/jvi.64.8.3590-3597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberger M., Pevear D., Diana G. D., Kuechler E., Blaas D. Stabilization of human rhinovirus serotype 2 against pH-induced conformational change by antiviral compounds. J Gen Virol. 1991 Feb;72(Pt 2):431–433. doi: 10.1099/0022-1317-72-2-431. [DOI] [PubMed] [Google Scholar]
- Guttman N., Baltimore D. A plasma membrane component able to bind and alter virions of poliovirus type 1: studies on cell-free alteration using a simplified assay. Virology. 1977 Oct 1;82(1):25–36. doi: 10.1016/0042-6822(77)90029-0. [DOI] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J Cell Biol. 1993 Oct;123(1):89–97. doi: 10.1083/jcb.123.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. The preendosomal compartment comprises distinct coated and noncoated endocytic vesicle populations. J Cell Biol. 1991 May;113(4):731–741. doi: 10.1083/jcb.113.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A. Unpacking the incoming influenza virus. Cell. 1992 May 15;69(4):577–578. doi: 10.1016/0092-8674(92)90219-3. [DOI] [PubMed] [Google Scholar]
- Heuser J., Zhu Q., Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J Cell Biol. 1993 Jun;121(6):1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer F., Gruenberger M., Kowalski H., Machat H., Huettinger M., Kuechler E., Blaas D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover-Litty H., Greve J. M. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J Virol. 1993 Jan;67(1):390–397. doi: 10.1128/jvi.67.1.390-397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Freistadt M. S., Racaniello V. R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990 Oct;64(10):4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Peters D., Racaniello V. R. Poliovirus mutants resistant to neutralization with soluble cell receptors. Science. 1990 Dec 14;250(4987):1596–1599. doi: 10.1126/science.2177226. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Stukenbrok H., Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Yin F. H., Noble-Harvey J. Fractionation of biologically active and inactive populations of human rhinovirus type 2. Virology. 1975 Feb;63(2):384–394. doi: 10.1016/0042-6822(75)90311-6. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kronenberger P., Vrijsen R., Boeyé A. Chloroquine induces empty capsid formation during poliovirus eclipse. J Virol. 1991 Dec;65(12):7008–7011. doi: 10.1128/jvi.65.12.7008-7011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger P., Vrijsen R., Boeyé A. Compartmentalization of subviral particles during poliovirus eclipse in HeLa cells. J Gen Virol. 1992 Jul;73(Pt 7):1739–1744. doi: 10.1099/0022-1317-73-7-1739. [DOI] [PubMed] [Google Scholar]
- Kronenberger P., Vrijsen R., Geerts A., Boeyé A. Internalization of intact poliovirus by HeLa cells as shown by subcellular fractionation in isoosmotic Nycodenz gradients. J Gen Virol. 1992 Mar;73(Pt 3):597–605. doi: 10.1099/0022-1317-73-3-597. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Monroe S. S., Rueckert R. R. Role of maturation cleavage in infectivity of picornaviruses: activation of an infectosome. J Virol. 1993 Apr;67(4):2110–2122. doi: 10.1128/jvi.67.4.2110-2122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Butterworth B. E. Investigation of the structure of polio- and human rhinovirions through the use of selective chemical reactivity. Virology. 1976 May;71(1):207–216. doi: 10.1016/0042-6822(76)90106-9. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Shimshick E. J. Interaction of liposomes with subviral particles of poliovirus type 2 and rhinovirus type 2. J Virol. 1976 Aug;19(2):746–749. doi: 10.1128/jvi.19.2.746-749.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Noble-Harvey J. Comparison of in vitro and cell-mediated alteration of a human Rhinovirus and its inhibition by sodium dodecyl sulfate. J Virol. 1973 Oct;12(4):819–826. doi: 10.1128/jvi.12.4.819-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R. The structure and replication of rhinoviruses. Curr Top Microbiol Immunol. 1982;97:1–26. doi: 10.1007/978-3-642-68318-3_1. [DOI] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Different pH requirements for entry of the two picornaviruses, human rhinovirus 2 and murine encephalomyocarditis virus. Virology. 1984 Dec;139(2):346–357. doi: 10.1016/0042-6822(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Madshus I. H., Sandvig K., Olsnes S., van Deurs B. Effect of reduced endocytosis induced by hypotonic shock and potassium depletion on the infection of Hep 2 cells by picornaviruses. J Cell Physiol. 1987 Apr;131(1):14–22. doi: 10.1002/jcp.1041310104. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Schmid S., Kern H., Harms E., Male P., Mellman I., Helenius A. Rapid analytical and preparative isolation of functional endosomes by free flow electrophoresis. J Cell Biol. 1987 Apr;104(4):875–886. doi: 10.1083/jcb.104.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. W., Baxt B., Brown F., Harber J., Murdin A., Wimmer E. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology. 1993 Feb;192(2):568–577. doi: 10.1006/viro.1993.1073. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Moscufo N., Yafal A. G., Rogove A., Hogle J., Chow M. A mutation in VP4 defines a new step in the late stages of cell entry by poliovirus. J Virol. 1993 Aug;67(8):5075–5078. doi: 10.1128/jvi.67.8.5075-5078.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer C., Frasel L., Kuechler E., Blaas D. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology. 1987 May;158(1):255–258. doi: 10.1016/0042-6822(87)90264-9. [DOI] [PubMed] [Google Scholar]
- Noble-Harvey J., Lonberg-Holm K. Sequential steps in attachment of human rhinovirus type 2 to HeLa cells. J Gen Virol. 1974 Oct;25(1):83–91. doi: 10.1099/0022-1317-25-1-83. [DOI] [PubMed] [Google Scholar]
- Olsnes S., van Deurs B., Sandvig K. Protein toxins acting on intracellular targets: cellular uptake and translocation to the cytosol. Med Microbiol Immunol. 1993 May;182(2):51–61. doi: 10.1007/BF00189373. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Roberts L. M., Lord J. M. Toxin entry: how reversible is the secretory pathway? Trends Cell Biol. 1992 Jul;2(7):183–185. doi: 10.1016/0962-8924(92)90230-k. [DOI] [PubMed] [Google Scholar]
- Pérez L., Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J Virol. 1993 Aug;67(8):4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff C. F., Fuchs R., Mellman I., Robbins A. R. Chinese hamster ovary cell mutants with temperature-sensitive defects in endocytosis. I. Loss of function on shifting to the nonpermissive temperature. J Cell Biol. 1986 Dec;103(6 Pt 1):2283–2297. doi: 10.1083/jcb.103.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Schmid S., Fuchs R., Kielian M., Helenius A., Mellman I. Acidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutants. J Cell Biol. 1989 Apr;108(4):1291–1300. doi: 10.1083/jcb.108.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe D. M., Jesurum A., Murphy R. F. Absence of Na+,K(+)-ATPase regulation of endosomal acidification in K562 erythroleukemia cells. Analysis via inhibition of transferrin recycling by low temperatures. J Biol Chem. 1991 Feb 25;266(6):3469–3474. [PubMed] [Google Scholar]
- Skern T., Sommergruber W., Blaas D., Pieler C., Kuechler E. Relationship of human rhinovirus strain 2 and poliovirus as indicated by comparison of the polymerase gene regions. Virology. 1984 Jul 15;136(1):125–132. doi: 10.1016/0042-6822(84)90253-8. [DOI] [PubMed] [Google Scholar]
- Tooze J., Hollinshead M. Tubular early endosomal networks in AtT20 and other cells. J Cell Biol. 1991 Nov;115(3):635–653. doi: 10.1083/jcb.115.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., Marsh M. Endocytosis: what goes in and how? J Cell Sci. 1992 Sep;103(Pt 1):1–8. doi: 10.1242/jcs.103.1.1a. [DOI] [PubMed] [Google Scholar]
- Werner G., Hagenmaier H., Drautz H., Baumgartner A., Zähner H. Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity. J Antibiot (Tokyo) 1984 Feb;37(2):110–117. doi: 10.7164/antibiotics.37.110. [DOI] [PubMed] [Google Scholar]
- Wetz K., Kucinski T. Influence of different ionic and pH environments on structural alterations of poliovirus and their possible relation to virus uncoating. J Gen Virol. 1991 Oct;72(Pt 10):2541–2544. doi: 10.1099/0022-1317-72-10-2541. [DOI] [PubMed] [Google Scholar]
- Willingmann P., Barnert H., Zeichhardt H., Habermehl K. O. Recovery of structurally intact and infectious poliovirus type 1 from HeLa cells during receptor-mediated endocytosis. Virology. 1989 Feb;168(2):417–420. doi: 10.1016/0042-6822(89)90286-9. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984 Jul;37(3):789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Zeichhardt H., Wetz K., Willingmann P., Habermehl K. O. Entry of poliovirus type 1 and Mouse Elberfeld (ME) virus into HEp-2 cells: receptor-mediated endocytosis and endosomal or lysosomal uncoating. J Gen Virol. 1985 Mar;66(Pt 3):483–492. doi: 10.1099/0022-1317-66-3-483. [DOI] [PubMed] [Google Scholar]




