Summary
-
1
Environmental risk assessment of contaminants is conventionally based on toxic effects assessed in organism‐level test systems. We suggest that, for the prediction of toxicant effects, population‐ and community‐level effects should be considered. The aim of this study was to investigate how predation could alter a prey population's response to a toxicant to reveal effects at population and community levels.
-
2
Populations of the brine shrimp Artemia sp. were maintained in the laboratory with and without simulated predation. Individuals were exposed for 1 h to the pyrethroid insecticide esfenvalerate (0, 0·01, 0·04 and 0·08 µg L−1) and subsequently observed for 6 weeks.
-
3
Unpredated exposed populations showed a reduced population density compared with the control. However, even at the highest concentration of insecticide, populations were sustained until the end of the experiment. The lower density in the exposed populations led to reduced competition and subsequently to enhanced development of surviving individuals and an increased proportion of young individuals. In contrast, the combination of predation and short‐term toxicant exposure at concentrations of 0·04 and 0·08 µg L−1 produced extinction of the populations after 39 and 32 days of exposure, respectively.
-
4
Synthesis and applications. The response of populations of brine shrimp to toxicants at the community level may be stronger when predation is present than the response of populations without predation pressure, as the regulation capacity of the population (measured as an increased production of offspring at reduced population densities) is exhausted when predation is present. Future ecotoxicological risk assessment schemes should consider relevant community characteristics such as predation as part of an environmental risk assessment.
Keywords: community level, pesticide, population regulation, predation, toxicant stress
Introduction
An increasing number of ecotoxicological studies are devoted to population‐level effects because environmental risk assessment based merely on toxic effects of contaminants at the individual level may not predict effects at higher ecological levels. Theoretical and empirical studies have demonstrated that at the population level density‐dependent processes as a result of competition may modify toxicant effects. These studies commonly find that the population‐level effects do not follow the usual pattern of concentration–response relationship: the potential for density‐dependent compensations is highest when the toxicant dose is strong enough to reduce density, but not strong enough to affect all individuals. Hence, at modest toxicant concentrations, high‐density populations can show a compensatory response to the toxicant. Examples include polychaetes exposed to fluoranthene (Linke‐Gamenick, Forbes & Sibly 1999), mysids exposed to para‐nonylphenol (Kuhn et al. 2001), daphnids exposed to cadmium (Barata, Baird & Soares 2002) and fenvalerate (Liess, Pieters & Duquesne 2006), trichopterans exposed to fenvalerate (Liess 2002) and ephemeropterans exposed to esfenvalerate (Beketov & Liess 2005).
Although there are numerous studies devoted to direct and indirect toxicant effects on communities, for example trophic cascades, taxonomic shifts, functional alterations and behavioural effects (reviewed by Brock & Budde 1994; Fleeger, Carman & Nisbet 2003), much less is known about density‐dependent interactions at the community level and, to the best of our knowledge, there are no studies investigating toxicant effects on density‐dependent interactions in populations subjected to predator pressure. Nevertheless, it is obvious that predation could alter sensitivity of the prey by reducing their density and intraspecific competition. In addition, toxicants can increase the vulnerability of prey at the individual level. Examples include reduced survival of Limnephilus lunatus (Trichoptera) contaminated with particle‐bound fenvalerate in the presence of gammarids (Schulz & Liess 2001) and of Baetis sp. (Ephemeroptera) exposed to azinphos‐methyl and fenvalerate in presence of predatory fish (Schulz & Dabrowski 2001) and reduced survival of spiders Oedothorax apicatus exposed to deltamethrin as a result of predation by carabid beetles (Everts et al. 1991). The common pattern in all these examples is that organisms exposed to a toxicant are more vulnerable to predation than the unexposed organisms.
The aim of the present study was to investigate how predation could alter the response of prey populations to a toxicant by exhausting its regulation capacity (based on an increased production of offspring at reduced population densities of the prey population). We aimed to establish the level at which increased mortality (induced by the toxicant and by predation) could be compensated for by toxicant‐reduced population growth. Finally, we sought to enhance our understanding of the process by which toxicants propagate from the population level to the community level.
Materials and methods
Populations of brine shrimp Artemia sp. were maintained in the laboratory over a period of 4·5 months. Commencing from the second month of culturing, half of the populations were randomly selected for treatment with predation simulation. Approximately 10% of these animals were removed weekly. After 3 months, the Artemia sp. populations were exposed to pulse (1‐h) contamination with the insecticide esfenvalerate. After exposure and subsequent rinsing of the tested animals, the populations were maintained for a further 46 days. During this time population density and size of organisms were monitored weekly.
test organisms
Dry Artemia sp. cysts were collected from Kulundinskoe Lake (Altaiskii Kray, Russia). Although it has been proposed that all Artemia populations in south Siberia be unified as a polymorphous species Artemia sibirica (Smirnov 1966), we designated the animals in our experiments as Artemia sp. as taxonomy of the Artemia genus in Siberia is not yet sufficiently well defined. Under laboratory conditions, Artemia sp. populations had an ovoviviparous type of reproduction (producing free‐swimming nauplii). The observed female reproductive period was 1–2 weeks, and normally one, on rare occasions two, broods were produced by each female.
Cultivation of 32 Artemia sp. populations commenced at the beginning of December 2004. Each population initially consisted of 100 second‐instar larvae. This number was chosen to ensure that populations were close to carrying capacity at the beginning of the observation period. Populations were maintained in plastic indoor microcosms containing 0·3 L cultivation water. Animals were fed with dried pressed Spirulina platensis algae (produced by NPO Ekologiya pitaniya, Moscow, Russia), macerated in distilled water and homogenized before feeding. Cultivation water with a salinity of 35 was prepared by mixing synthetic sea salt (Sera GmbH, Heinsburg, Germany) with distilled water. Half of the water in each test vessel was renewed once every 2 weeks and organic particles deposited on the bottom of vessels were removed.
Predation simulation was performed by collecting the animals by pipette. Every week, four animals of each generation were removed from each predated population. The number of animals removed was reduced to one individual per generation whenever at least one of the populations contained fewer than 10 individuals of this particular generation. This scheme was feasible as not more than three generations were present in a vessel at once. The generations could be distinguished according to size and development stage.
It is generally accepted that a predator's density is dependent on the density of its prey, and a decrease (or increase) in predator density follows a decrease (or increase) in density of the prey after a time delay (Begon, Harper, & Townsend 1996). Thus predation pressure changes within certain limits because of the delayed response of the predator: delayed density dependence (Varley 1947). Predation simulation in this study was designed to reflect broadly this type of dependence.
In nature, delay in the predator's response to prey density and the subsequent change in predation pressure depends on the reproductive rate of the predator and other factors, and thus can vary widely (Begon, Harper, & Townsend 1996). Hence extrapolations of the results of this study to natural systems should be made with care.
Populations of Artemia sp. were maintained on a stable food regime for a period of 3·5 months (five or six generations of Artemia sp.) before exposure to a toxicant, during which time they approached an equilibrium state. Predation simulation was performed during the 2‐month period before toxicant exposure to stabilize the predated populations. At the end of this time, the populations were relatively stable with a uniform density, as estimated by qualitative observation.
short‐term exposure of organisms
Contamination was carried out on 15 March 2005 in glass Petri dishes. Esfenvalerate ((S)‐alpha‐cyano‐3‐phenoxybenzyl(S)‐2‐(−4‐chlorophenyl)‐3‐methylbutyrate) was applied as an emulsifiable concentrate (Sumi‐alfa®, Sumitomo, Osaka, Japan; produced and packed by ZAO Avgust, Moscow, Russia) containing 50 g L−1 active ingredient. All concentrations given refer to the active ingredient. The solutions were prepared from the cultivation water and the emulsifiable concentrate. The chemical parameters of cultivation water were measured before preparation of the toxicant solutions (Table 1). All the analyses were carried out by the Western Siberian Center for Environmental Monitoring (Novosibirsk, Russia), by standard methods (Semenova 1977). The emulsifiable concentrate was used rather than the pure substances (technical grade) to approximate conditions in the field. The Artemia sp. populations were exposed to the toxicant for 1 h at the following nominal concentrations: control, 0·01, 0·05 and 0·1 µg L−1. The concentrations were obtained by serial dilution of a stock solution containing 1 µg L−1. Larvae were rinsed after exposure with pesticide‐free water.
Table 1.
Hydrochemistry of the cultivation water measured before preparation of the toxicant solutions
| Parameter | Value |
|---|---|
| Total ammonia (mg L−1) | 0·04 |
| Nitrite (mg L−1) | < 0·0001 |
| Nitrate (mg L−1) | 0·06 |
| Orthophosphate (mg L−1) | 0·03 |
| Hardness (mg CaCO3 L−1) | 162·2 |
| BOD5 (mg L−1) | 1·35 |
| pH | 7·75 |
Real exposure concentrations were measured using standard methods (Anonymous 2004). Liquid‐phase extraction of 1‐L volumes was carried out with hexane. Two litres of each exposure solution were prepared; one was used for the exposure and the other was extracted for analyses. This prevented a decrease in measured concentrations resulting from insecticide adsorption by the animals’ bodies. The measurements were made with a gas chromatograph (Tsvet 550 C; OKBA, Dzerzhinsk, Russia) supplied with an integral microelectron capture detector. Measurement of each exposure solution was made once. For the nominal concentrations 0·01, 0·05 and 0·1 µg L−1, the measured values were 0·01, 0·04 and 0·08 µg L−1, respectively. Concentrations referred to below are measured values.
After exposure the Artemia sp. populations were monitored using a digital camera (Olympus C‐160; Olympus Corporation, Tokyo, Japan). Each population was photographed once per week and the number and size (from front of head to end of tail, in millimetres) of individuals were measured. Generations were distinguished by their size and the development stage of individuals. Statistical analyses were performed using STATISTICA® 5·5 A (StatSoft, Tulsa, OK) software. The significance of differences of means was examined using one‐way anova with post‐hoc tests for Fisher's protected least‐significant difference (LSD).
Results
Artemia sp. populations were monitored 3 days before and 4, 11, 18, 25, 32, 39 and 46 days after exposure. The combination of simulated predation and chronic toxicant effect at concentrations of 0·04 and 0·08 µg L−1 resulted in extinction of the populations at 39 and 32 days after exposure, respectively (Fig. 1a). In contrast, unpredated populations at the same toxicant concentrations survived with a reduced density (Fig. 1b).
Figure 1.
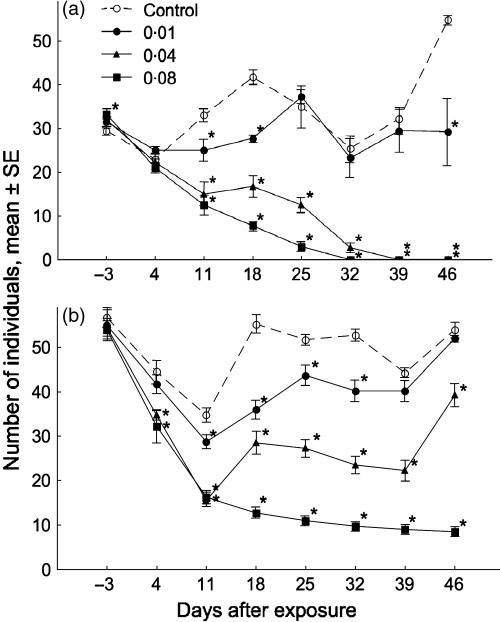
Mean densities of Artemia sp. populations before and after 1 h of exposure to esfenvalerate (control, 0·01, 0·04, 0·08 µg L−1) with (a) and without (b) simulated predation. Asterisks indicate significant (analyses of variance, LSD test, P < 0·005) differences from the control series at the respective time of observation.
The oldest generation (extant 3 days before the exposure) was termed the G1 generation and subsequent generations were numbered consecutively. At the moment of exposure all the populations consisted of two generations (G1 and G2; Fig. 2). Simulated predation resulted in an increase in developmental rate. Thus in the control and at 0·01 and 0·04 µg L−1 toxicant concentrations, the G3 generation (offspring of G1) appeared in the predated populations at 11 days after exposure, but in the unpredated series this G3 generation started to appear only after 18 days. Esfenvalerate at a concentration of 0·08 µg L−1 caused early reproduction by the G1 generation, and therefore the G3 generation occurred immediately after exposure in the predated and unpredated populations (Fig. 2).
Figure 2.
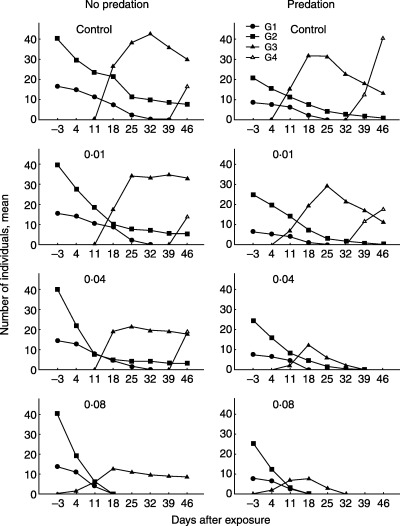
Mean densities of different generations (G1, G2, G3 and G4) of Artemia sp. before and after 1 h of exposure to esfenvalerate (control, 0·01, 0·04, 0·08 µg L−1) with and without simulated predation.
The abundance of the young generations (G3 and G4) was negatively correlated with esfenvalerate concentrations (Fig. 3). In the unpredated populations, the proportion of younger generations (G3 and G4) increased with the increase in toxicant concentration, whereas their abundance did not significantly change in the predated populations. However, for the predated populations this comparison could be made only for the control and lowest exposed populations (0·01 µg L−1) because of mortality at higher exposure concentrations. The densities of these generations expressed as a percentage of the total population density are shown in Fig. 4.
Figure 3.
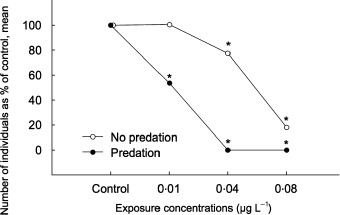
Mean densities of G3 and G4 generations (in sum) of Artemia sp. expressed as a percentage of respective controls in different experimental series (unpredated and predated; control, 0·01, 0·04, 0·08 µg L−1) at the end of the experiment (46 days after exposure) (number of individuals, means, %). Asterisks indicate significant (analyses of variance, LSD test, P < 0·005) differences from the control series at the respective time of observation.
Figure 4.
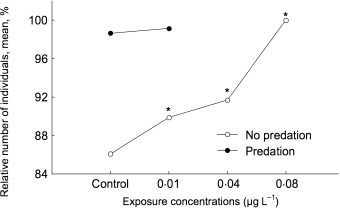
Mean densities of G3 and G4 generations (in sum) of Artemia sp. expressed as a percentage of respective total densities of different experimental series (unpredated and predated; control, 0·01, 0·04, 0·08 µg L−1) at the end of the experiment (46 days after exposure) (number of individuals, means, %). Asterisks indicate significant (analyses of variance, LSD test, P < 0·005) differences from the control series at the respective time of observation.
In spite of the fact that predation caused an increase in developmental rate as a result of a decreased density, it did not cause an increase in final (maximum) body size of the adult organisms (control series, G1 generations; Fig. 5). A significant effect of the toxicant on body size was detected in the unpredated populations exposed to 0·08 µg L−1 of esfenvalerate. In this population, at the end of the experiment (46 days after exposure) the mean body size of the G3 generations was significantly higher (P < 0·001, LSD test) than the G3 generations in the respective control as well as all other unpredated populations exposed to esfenvalerate (concentrations 0·01 and 0·04 µg L−1; Fig. 5).
Figure 5.
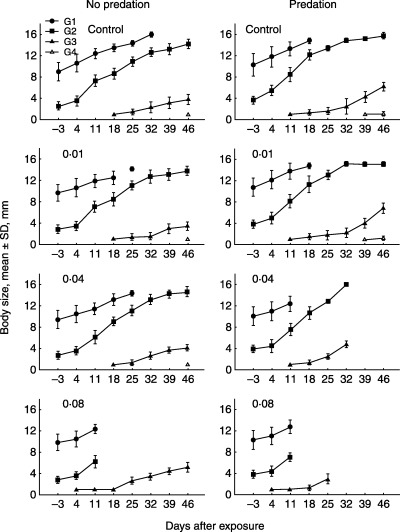
Mean body sizes of different generations (G1, G2, G3 and G4) of Artemia sp. before and after 1 h of exposure to esfenvalerate (control, 0·01, 0·04, 0·08 µg L−1) with and without simulated predation (mean ± SD, mm).
Discussion
The chronic effect of short‐term contamination with toxicants such as pyrethroids has been documented for various aquatic organisms (Liess & Schulz 1996; Liess 2002; Cold & Forbes 2004; Beketov & Liess 2005; Forbes & Cold 2005; Reynaldi & Liess 2005). The level of toxicant concentrations causing chronic effects in the present study is comparable with those found for other aquatic insects and crustaceans (Liess 2002; Cold & Forbes 2004; Beketov & Liess 2005; Reynaldi & Liess 2005). The combined effect of predation and exposure to toxicants has been studied previously (Clements, Cherry & Cairns 1989; Everts et al. 1991; Taylor et al. 1995; Schulz & Dabrowski 2001). However, these studies did not focus on how predator pressure resulting in a decreased regulation capacity (i.e. increased production of offspring at reduced population densities) of the prey population would alter the sensitivity of the prey to toxicant exposure at the population level. Previous studies have reported that individuals exposed to a toxicant are more vulnerable to predation than unexposed individuals. In contrast, in the present study reduced fitness of exposed individuals was not responsible for the combined effect of predation and toxicant exposure.
Studies focused on density‐dependent responses of populations to toxicants have shown that a decrease in density of animals exposed to a toxicant can be compensated for, because the surviving individuals experience more favourable conditions as a result of the reduced population density (Linke‐Gamenick, Forbes & Sibly 1999; Sibly, Williams & Jones 2000; Kuhn et al. 2001; Barata, Baird & Soares 2002; Liess 2002; Beketov & Liess 2005; Liess, Pieters & Duquesne 2006). In the present study this type of density‐dependent compensation was found for the unpredated series exposed to 0·08 µg L−1 of esfenvalerate. Thus a direct effect of the insecticide resulted in elimination of the G1 and G2 generations and a reduction in the density of the G3 generation (Fig. 2; unpredated, 0·08 µg L−1). Therefore, surviving individuals of the G3 generation had a relatively abundant food supply because of the absence of competitors, and were characterized by a significantly larger body size than their respective controls as well populations exposed to lower esfenvalerate concentrations. Obviously the increase in body size was achieved by relatively faster development of the organisms. A similar increase in development rate was caused by predation, but only in the control series (2, 5).
An analysis of population structure revealed that an increase in the proportion of relatively young G3 and G4 generations in the total population was positively correlated with increasing toxicant concentration (Fig. 4; unpredated series). This evidently resulted from a reduction in competition between individuals as the density was reduced by the toxicant (1, 2). This reduced density post‐exposure was more pronounced in the comparatively older G1 and G2 generations, which were directly exposed to the toxicant.
Through the inclusion of predation, this experiment has demonstrated that density‐dependent compensation for the toxicant effect was not possible under the pressure of constant predation. Predation simulation in the control series resulted in faster individual development through a decrease in population density, but in the exposed series this decrease in density exhausted the populations’ potential to recover. Thus, low density itself is a favourable factor that facilitates development of organisms. However, the factors that cause this low density (as predation or a toxicant) may in themselves be fatal for the maintenance of the population. Mechanisms of density‐dependent compensation of a toxicant effect demonstrated previously for various organisms at the population level (Linke‐Gamenick, Forbes & Sibly 1999; Kuhn et al. 2001; Barata, Baird & Soares 2002; Liess 2002; Beketov & Liess 2005; Liess, Pieters & Duquesne 2006) may not act with the same magnitude at the community level when predator–prey interaction is present.
We conclude that the response of populations to toxicants at the community level, when predation is present, may be considerably stronger than that of populations without predation pressure, as regulation capacity is decreased as a result of predation. Predation is a significant factor in communities, hence future ecotoxicological risk assessment schemes aimed at predicting effects of toxicants at the community level should incorporate consideration of predation.
Acknowledgements
This research was financially supported by the European Union (European Commission, Marie Curie IIF contract No. MIF1‐CT‐2006‐021860).
Re‐use of this article is permitted in accordance with the Creative Commons Deed, Attribution 2·5, which does not permit commercial exploitation.
References
- Anonymous (2004) Guidance. Methods for Measurement of Synthetic Pyrethroids Deltamethrin, Fenvalerate, and Alpha‐cyperMethrin in Soil Samples. Methods of Measurement by Gas‐Liquid Chromatography. RD52·18·656‐2004. NPO Taifun, Moscow, Russia[in Russian]. [Google Scholar]
- Barata, C. , Baird, D.J. & Soares, A.M.V.M. (2002) Demographic responses of a tropical cladoceran to cadmium: effects of food supply and density. Ecological Application, 12, 552–564. [Google Scholar]
- Begon, M. , Harper, J.L. & Townsend, C.R. (1996) Ecology: Individuals, Populations and Communities, 3rd edn Blackwell Science, Oxford, UK. [Google Scholar]
- Beketov, M. & Liess, M. (2005) Acute contamination with esfenvalerate and food limitation: chronic effect on the mayfly, Cloeon dipterum . Environmental Toxicology and Chemistry, 24, 281–1286. [DOI] [PubMed] [Google Scholar]
- Brock, T.C.M. & Budde, B.J. (1994) On the choice of structural parameters and endpoints to indicate responses of freshwater ecosystems to pesticide stress Freshwater Field Tests for Hazard Assessment of Chemicals (eds I.R. Hill, F. Heimbach, P. Leeuwangh, P. Matthiessen), pp. 19–56. CRC Press Inc., Boca Raton, FL. [Google Scholar]
- Clements, W.H. , Cherry, D.S. & Cairns, J. Jr (1989) The influence of copper exposure on predator–prey interactions in aquatic insect communities. Freshwater Biology, 21, 483–488. [Google Scholar]
- Cold, A. & Forbes, V. (2004) Consequences of a short pulse of pesticide exposure for survival and reproduction of Gammarus pulex . Aquatic Toxicology, 67, 287–299. [DOI] [PubMed] [Google Scholar]
- Everts, J.W. , Willemsen, I. , Stulp, M. , Simons, L. , Aukema, B. & Kammenga, J. (1991) The toxic effect of deltamethrin on Linyphyid and Erigonid spiders in connection with ambient temperature, humidity and predation. Archives of Environmental Contamination and Toxicology, 20, 20–24. [DOI] [PubMed] [Google Scholar]
- Fleeger, J.W. , Carman, K.R. & Nisbet, R.M. (2003) Indirect effects of contaminants in aquatic ecosystems. Science of the Total Environment, 317, 207–233. [DOI] [PubMed] [Google Scholar]
- Forbes, V. & Cold, A. (2005) Effect of the pyrethroid esfenvalerate on life‐cycle traits and population dynamics of Chironomus riparius: importance of exposure scenario. Environmental Toxicology and Chemistry, 24, 78–86. [DOI] [PubMed] [Google Scholar]
- Kuhn, A. , Munns, W.R. , Champlin, D. , McKinney, R. , Tagliabue, M. , Serbst, J. & Gleason, T. (2001) Evaluation of the efficacy of extrapolation population modeling to predict the dynamics of Americamysis bahia populations in the laboratory. Environmental Toxicology and Chemistry, 20, 213–221. [PubMed] [Google Scholar]
- Liess, M. (2002) Population response to toxicants is altered by intraspecific interaction. Environmental Toxicology and Chemistry, 21, 138–142. [PubMed] [Google Scholar]
- Liess, M. & Schulz, R. (1996) Chronic effect of short‐term contamination with the pyrethroid insecticide fenvalerate on the caddisfly Limnephilus lunatus . Hydrobiologia, 324, 99–106. [Google Scholar]
- Liess, M. , Pieters, B. & Duquesne, S. (2006) Long‐term signal of population disturbance after pulse exposure to an insecticide: rapid recovery of abundance, persistent alteration of structure. Environmental Toxicology and Chemistry, 24, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Linke‐Gamenick, I. , Forbes, V.E. & Sibly, R.M. (1999) Density‐dependent effects of a toxicant on lifehistory traits and population dynamics of a capitellid polychaete. Marine Ecology Progress Series, 184, 139–148. [Google Scholar]
- Reynaldi, S. & Liess, M. (2005) Influence of duration of exposure to the pyrethroid fenvalerate on sublethal responses and recovery of Daphnia magna Straus. Environmental Toxicology and Chemistry, 24, 1160–1164. [DOI] [PubMed] [Google Scholar]
- Schulz, R. & Dabrowski, J.M. (2001) Combined effects of predatory fish and sublethal pesticide contamination on the behaviour and mortality of mayfly nymphs. Environmental Toxicology and Chemistry, 20, 2537–2543. [DOI] [PubMed] [Google Scholar]
- Schulz, R. & Liess, M. (2001) Runoff simulation with particle‐bound fenvalerate in multispecies stream microcosms: importance of biological interaction. Environmental Toxicology and Chemistry, 20, 757–762. [DOI] [PubMed] [Google Scholar]
- Semenova, A.D. (1977) Guide for Chemical Analyses of Surface Waters. Gidrometeoizdat, Leningrad, Russia[in Russian]. [Google Scholar]
- Sibly, R.M. , Williams, T.D. & Jones, M.B. (2000) How environmental stress affects density dependence and carrying capacity in a marine copepod. Journal of Applied Ecology, 37, 388–397. [Google Scholar]
- Smirnov, S.S. (1966) Phyllopods. Fauna of USSR, Vol. 4 Nauka, Moscow, Russia[in Russian]. [Google Scholar]
- Taylor, E.J. , Morrison, J.E. , Blockwell, S.J. , Tarr, A. & Pascoe, D. (1995) Effects of lindane on the predator prey interaction between Hydra oligactis Pallas and Daphnia magna Strauss. Archives of Environmental Contamination and Toxicology, 29, 291–296. [Google Scholar]
- Varley, G.C. (1947) The natural control of population balance in the knapweed gall‐fly (Urophora jeceana). Journal of Animal Ecology, 16, 139–187. [Google Scholar]
- Woin, P. (1998) Short‐ and long‐term effects of the pyrethroid insecticide fenvalerate on an invertebrate pond community. Ecotoxicology and Environmental Safety, 41, 137–156. [DOI] [PubMed] [Google Scholar]


