Abstract
The greatly increased tetramer strength of liganded fetal hemoglobin compared with adult hemoglobin is shown by its 70-fold smaller tetramer-dimer dissociation constant. This property has been shown previously to be only partially caused by the 5-amino-acid differences at both types of interfaces in each hemoglobin. A major contributor to tetramer strengthening is the 18-amino-acid N-terminal A helix of the γ-subunit of fetal hemoglobin, which differs from the β-subunit of adult hemoglobin at eight amino acid residues. This long-distance communication between the A helix and the distant C helix and FG helical corner comprising the subunit contacts at the allosteric interface represents internal signaling. Physiologically, its greater tetramer strength endows fetal hemoglobin with the capacity to abstract oxygen from maternal adult hemoglobin. It also leads to resistance of fetal red cells to the malaria parasite because the HbF tetramer does not dissociate to dimers as readily as HbA; dimers are digested by malaria proteases but tetramers are not. In this communication, we report which sites on the A helix of the γ-subunit are important for tetramer strengthening in HbF by substituting certain amino acids in the β-subunit by the corresponding residues in the γ-subunit. The recombinant hemoglobins containing up to five replacements together have been extensively characterized. Mass values were within 1 unit of theory. Gly 1 (γ) of HbF with its high pKa of 8.1 compared with a 7.1 value for Val 1 (β) of HbA creates a highly electropositive N terminus that may couple with the electronegative sequence just after it on the γ-subunit. The Leu 3 to Phe replacement has no apparent role; however, position 5 is important because replacement of Pro 5 (β) by Glu 5 (γ) promotes tetramer strengthening. The Glu → Asp replacement at position 7 enhances this effect because of the lower pKa of Asp but the Val → Ile substitution at position 11 has no effect. Thus, the three positive/negative sites at positions 1, 5, and 7 account for practically all of the tetramer strength of HbF, as illustrated by an electrostatic surface potential analysis. The pathway by which information is transmitted to the distant allosteric subunit interfaces is currently under study. Oxygen-binding properties of the hemoglobins with charged substitutions more closely resemble those of HbA rather than those of HbF. Thus, whereas the A helix has a major role in controlling the strength of interactions at the tetramer-dimer allosteric interface, oxygen-binding properties of HbA and HbF are influenced by sequences in the C helix and at the FG helical corner constituting the allosteric interface.
Keywords: Hemoglobin, long-range interaction, site-directed mutagenesis
In previous reports (Dumoulin et al. 1997,1998), the greatly increased strength of the fetal hemoglobin (HbF) tetramer in the liganded state (a 70-fold reduced tetramer-dimer dissociation constant [Kd] compared with that for HbA) was described. In this article, the term tetramer strength indicates the facility with which the contacts between the dimer pairs occur in forming a tetramer. Its mathematical representation is the dissociation (or association) constant in units of concentration. We prefer to use strength rather than stability because in the hemoglobin literature, lack of stability, for example, caused by a mutation, can mean loss of heme and denaturation of the protein.
The 5-amino-acid substitutions at both types of subunit interfaces (the mobile tetramer-dimer interface and the rigid dimer-monomer interface) were shown to account for only a fraction of this difference in tetramer strength. Subsequently, a major contribution to this effect was found to reside in the A helix of the γ-subunit, which differs from its counterpart in the β-subunit at eight of 18 amino acid residues. Thus, the results for the γ-β hybrid Hb Felix (Dumoulin et al. 1998) represents an example of a long-range interaction between separated regions of the tetramer because the A helix is distant from the tetramer-dimer contacts with α-subunit, that is, the C helix and the FG helical corner (Perutz 1989). In the present report, we describe the individual contributions of the A helix to the increased tetramer strength of HbF. A significant physiological effect of the increased tetramer strength of HbF is the resistance of fetal red cells to the malaria parasite (Shear et al. 1998). Therefore, understanding its origin is important. Another objective is to determine which substitutions are responsible for the difference in oxygen binding and release between adult and fetal hemoglobins, which represents a relevant physiological mechanism in facilitating oxygen transfer from maternal to fetal blood. For example, we recently reported (Chen et al. 2000) that the Glu/Asp substitution at position β-43 at the allosteric interface, which is distant from the DPG (2,3-diphosphoglycerate) binding site, and not the supposed His/Ser replacement at position β-143 at the DPG binding site itself, was a critical factor in controlling the increased oxygen affinity of HbF in the presence of DPG. In this article, we report how the replacements at the A helix affect oxygen affinity.
Results and Discussion
Substitutions in the A and B helices of the β-subunit
Using a highly sensitive gel filtration procedure coupled with comprehensive data analysis (Manning et al. 1996,1999; Dumoulin et al. 1997,1998), we found that HbA had a Kd of 0.68 μM and HbF had a Kd of 0.01 μM in their liganded forms at pH 7.5. Before that report, these hemoglobins were not considered to differ in their tetramer strengths. A comparison of the structures of deoxy HbF and deoxy HbA (Frier and Perutz 1977) does not indicate that the subunit contacts of the former are tighter than the latter. The structure of liganded HbF has not been solved. Therefore, a comparison of liganded HbF with liganded HbA is not possible at this time. As described above, the A helix of the γ-subunit contributed more to the tetramer strength of HbF (low Kd) than did its subunit interface amino acids. In the present report, we have focused on the six charge and hydrophobic differences between the A helix and the beginning of the B helix of the β-subunit between residues 1 and 22. In this sequence, there are 10 amino acid substitutions at the underlined residues shown below:
β V1 H L T P E E K S A10 V T A L W G K V N V20 D E ... .
γ G1 H F T E E D K A T10 I T S L W G K V N V20 E D ... .
The substitutions made in this report were at positions 1, 3, 5, 7, 11, 21, and 22; the remaining sites involving Ala, Ser, or Thr at positions 9, 10, and 13 were left unchanged. Charge substitutions were chosen because we found previously that the higher pKa of Gly 1(γ) in HbF compared with that of Val 1(β) in HbA (8.1 vs. 7.1) endowed Hb V1G(β) with increased tetramer strength and also that acetylation at this site in HbF1 loosened the subunit contacts significantly (Chen et al. 2000; Manning and Manning 2001). M.F. Perutz suggested that we investigate the L3F (β) and V11I (β) replacements based on his structural comparison of deoxy HbA and deoxy HbF in which A helix movements were first noted (Frier and Perutz 1977). We also included a Pro → Ala substitution at position 5 as a control, in addition to the normal Pro → Glu replacement. The V1G(β) substitution was included in some mutations.
Purification and characterization
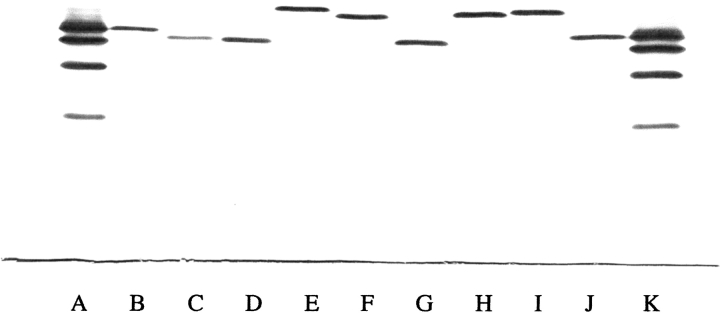
Each mutant Hb expressed in yeast and purified to homogeneity as described had a normal ultraviolet visible absorption spectrum in the CO form with no evidence of oxidation to met-Hb. Conversion to the oxy form for oxygen-binding studies (Manning 1981) proceeded smoothly without formation of oxidized forms. Isoelectric focusing of each purified mutant Hb (Fig. 1 ▶) showed that each was homogeneous and migrated to a position expected for the substitution(s).
Fig. 1.
Isoelectric focusing of purified recombinant Hb mutants. Approximately 5 μg of each Hb was applied to the pH 6.0–8.0 range gel (Hb Resolve, Isolab) and electrophoresed for 30 min at 600 V and 45 min at 900 V at 10°C. The gel was stained with the JB2 stain (Perkin Elmer Wallach). The anode is at the top and the cathode is at the bottom. (Lane A) Standard hemoglobins A, F, S, and C from top to bottom. (Lane B) Hb V11I (β). (Lane C) Hb L3F(β). (Lane D) Hb L3F(β)/V11I(β). (Lane E) Hb P5E(β). (Lane F) Hb V1G(β)/P5E(β). (Lane G) Hb V1G(β)/P5A(β). (Lane H) Hb V1G(β)/P5E(β)/E7D(β). (Lane I) Hb V1G(β)/P5E(β)/E7D(β)/D21E(β)/E22D(β). (Lane J) HbA. (Lane K) Standard hemoglobins as in lane A.
Circular dichroism
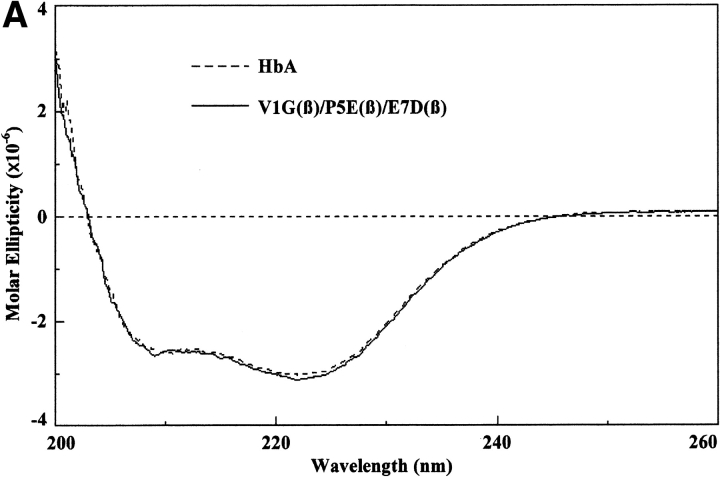
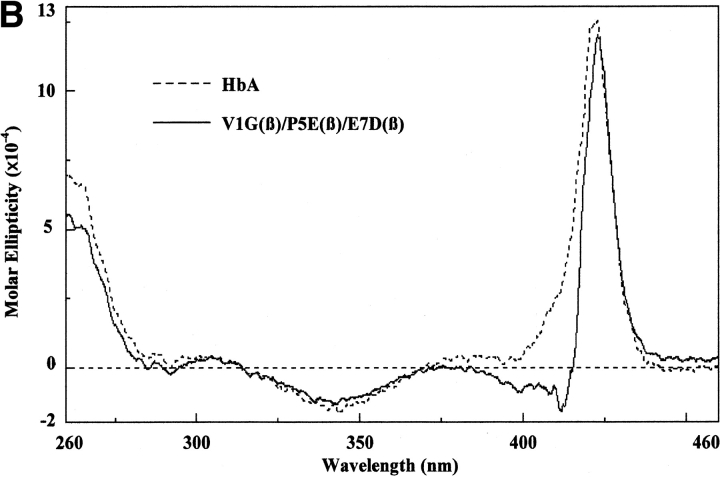
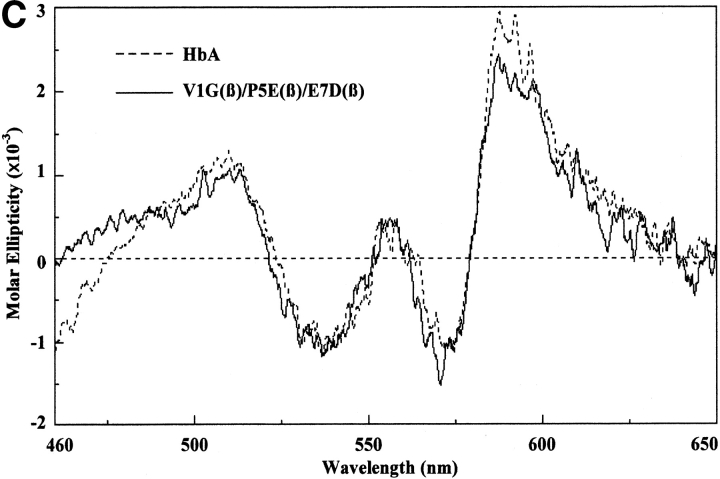
These measurements were performed as described by Martin de Llano and Manning (1994) for HbA and HbS but using a Jasco 715 instrument. The circular dichroism spectra of the recombinant mutants that have been reported for the first time in this communication have been measured in the visible, near ultraviolet, and far ultraviolet regions. Each gave the same pattern as HbA and HbS except for very minor differences; a typical spectrum is that for liganded V1G(β)/P5E(β)/E7D(β) shown in Figure 2 ▶. The region from 200 to 260 nm in the far ultraviolet, which is caused by the globin portion, is identical to those for HbA and HbS and for the triple mutant, indicating that there are no detectable global changes as a result of the mutations, expression, and purification procedures. The spectra of the near ultraviolet (260–460 nm) and the visible regions (460–650 nm), which derive from the heme moiety, are very close to those for HbA and HbS except for small differences. We have reported previously similar small differences when comparing natural and recombinant HbS and HbA (Martin de Llano and Manning 1994). The liganded triple mutant shown in Figure 2 ▶ has a much lower Kd than the values for these hemoglobins, so it is possible that some of the small circular dichroism changes observed are caused by that property.
Fig. 2.
Circular dichroism spectra. The circular dichroism spectra were recorded as described in the text. For the far ultraviolet light region (A), the Hb concentration was 10 μM as tetramer, and a 0.1-cm curvette was used for four scans. For the near ultraviolet light region (B), the Hb concentration was 30 μM as tetramer, and a 0.1-cm cuvette was used for eight scans. For the visible region (C), the Hb concentration was 30 μM as tetramer, and a 1.0-cm cuvette was used for 16 scans. Molar ellipticity was calculated on a heme basis for which the A539 was used.
Mass spectrometry
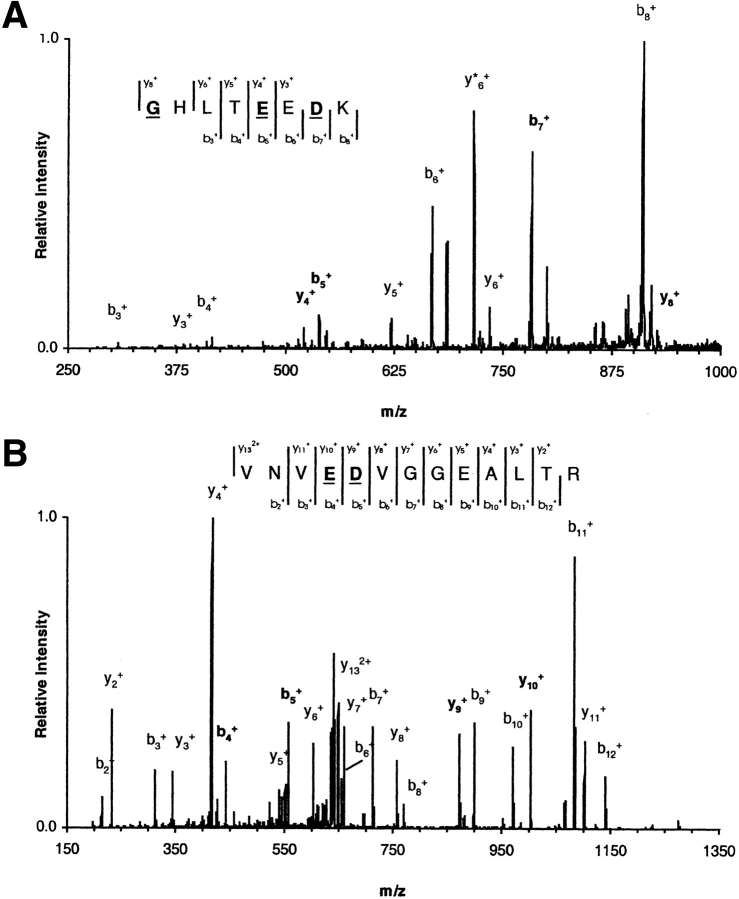
After the final fast protein liquid chromatography purification, hemoglobin characterization was performed by mass spectrometry (MS) at three different levels. Initially, the average molecular masses for the α and β chains were obtained by electrospray ionization/mass spectrometry (ESI/MS). The measured masses for all mutants were in agreement with the expected molecular masses derived from the corresponding sequence information (Table 1) to within 40 ppm. At a second level, tryptic peptide maps were obtained by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF)/MS in both positive and negative ionization modes. The peptide maps accounted for combined sequence coverage of at least 98% for each γ-β hybrid globin with mass accuracies within 50 ppm (results not shown). Regions not accounted for in the peptide maps correspond to the C-terminal dipeptides YR in the α chains and YH in the β chains. These dipeptides were later identified by ESI/MS, yielding a combined coverage of 100% for each globin. Finally, further evidence for the correct expression of each recombinant hemoglobin was obtained by mass spectrometric fragmentation of the peptide(s) containing the amino acid substitutions. The example shown (Fig. 3 ▶) is for the pentuple mutant. The correct substitutions were established for this and all other mutants. The fragmentation spectra yielded b- and y-ion series that enabled identification of the substituted amino acids from each peptide precursor ion. In Figure 3 ▶, the underlined residues refer to the correct amino acid replacements.
Table 1.
Measured average molecular masses of recombinant hemoglobin α, β, and γ-β hybrid subunits
| α-Chain | β-Chain | |||
| Recombinant hemoglobin | Measured massa (u) | ΔMb (u) | Measured massa (u) | ΔMb (u) |
| L3F | 15,126.7 ± 0.2 | 0.4 | 15,900.8 ± 0.1 | −0.4 |
| V11I | 15,126.9 ± 0.6 | 0.6 | 15,881.2 ± 0.2 | 0.0 |
| L3F/V11I | 15,126.3 ± 0.2 | 0.0 | 15,914.6 ± 0.1 | −0.6 |
| P5E | 15,126.6 ± 0.1 | 0.3 | 15,898.7 ± 0.2 | −0.5 |
| V1G/P5E | 15,126.8 ± 0.2 | 0.5 | 15,856.9 ± 0.5 | −0.2 |
| V1G/P5A | 15,126.6 ± 0.2 | 0.3 | 15,799.0 ± 0.0 | −0.1 |
| V1G/P5E/E7D | 15,126.5 ± 0.1 | 0.2 | 15,842.5 ± 0.2 | −0.6 |
| V1G/P5E/E7D/D21E/E22D | 15,126.3 ± 0.1 | 0.0 | 15,842.5 ± 0.1 | −0.6 |
a Average values from 10 independent measurements (AVG ± SEM, n = 10).
b Mass error; expected theoretical average mass (from sequence information) subtracted from measured mass.
Fig. 3.
Mass spectrometric analysis of pentuple mutant. ESI/MS/MS fragmentation spectra for peptides βT[1–8] (A) and βT[18–30] (B) from recombinant hemoglobin V1G/P5E/E7D/D21E/E22D. Precursor ions were selected from the total tryptic digest. The singly charged ion of peptide βT[1–8] was fragmented with 30% relative collisional energy (Finnigan's nomenclature) and the doubly charged ion of peptide βT[18–30] with 27%. Mass spectrometric fragmentation yielded the b- and y-ion series shown in the figure. Underlined residues correspond to the amino acid substitutions introduced in the β-chain sequence.
Tetramer-dimer dissociation constants (Kd values)
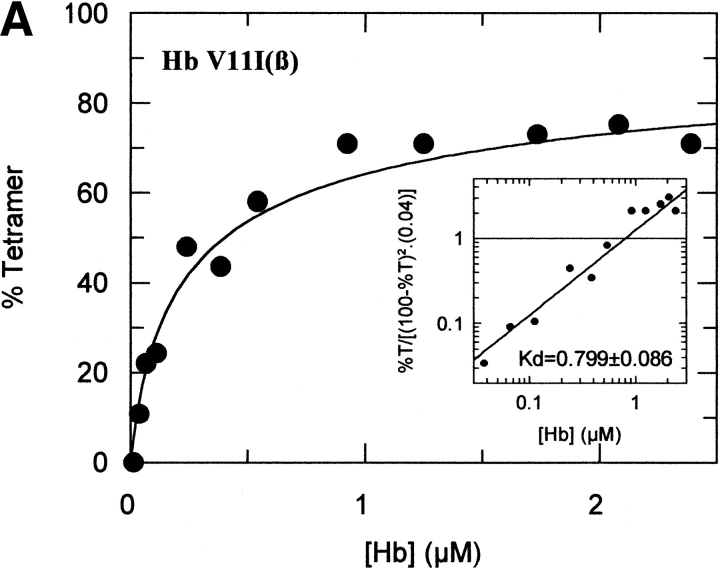
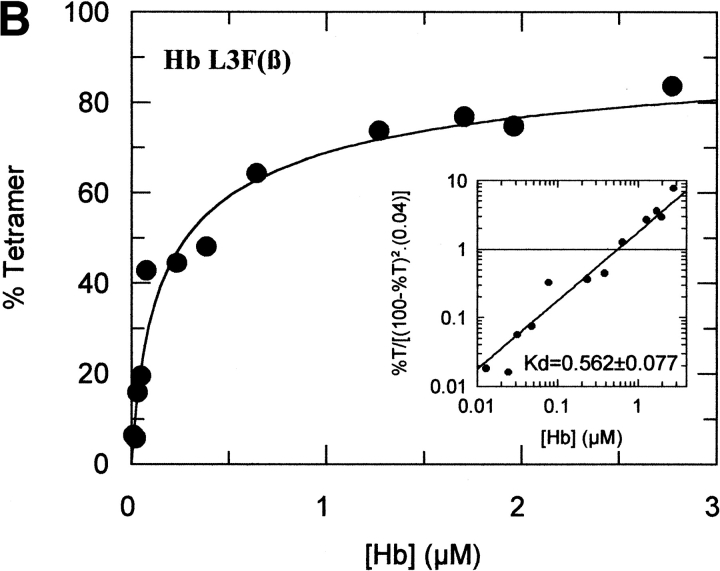
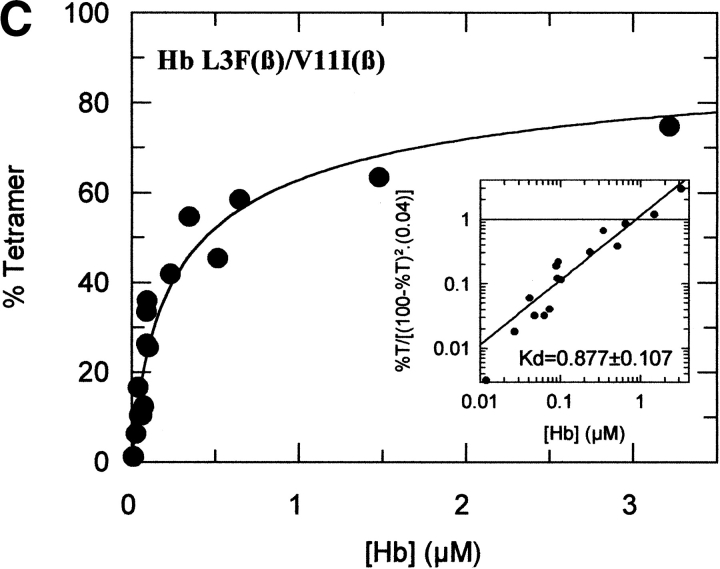
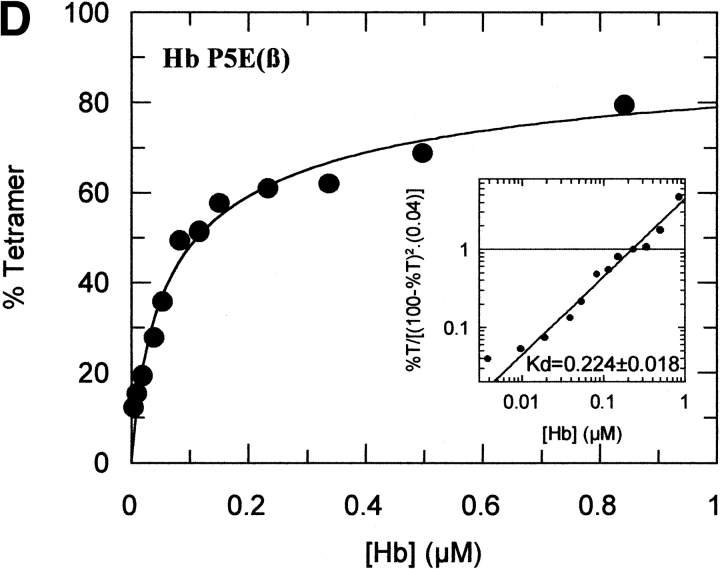
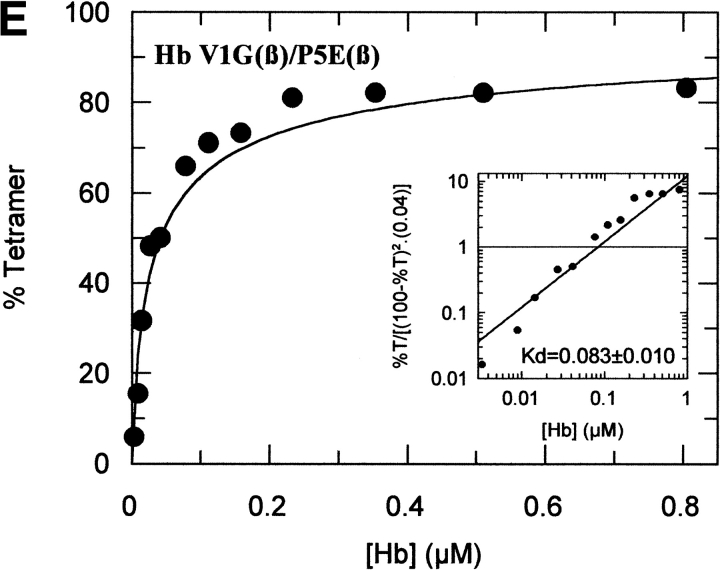
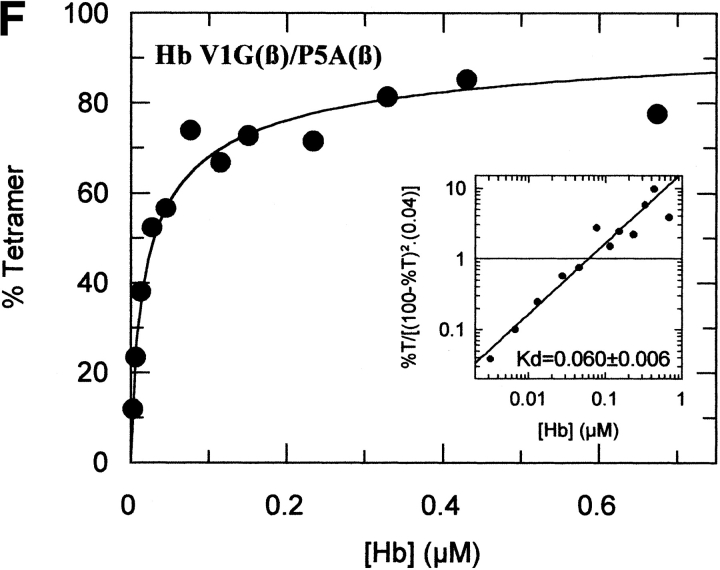
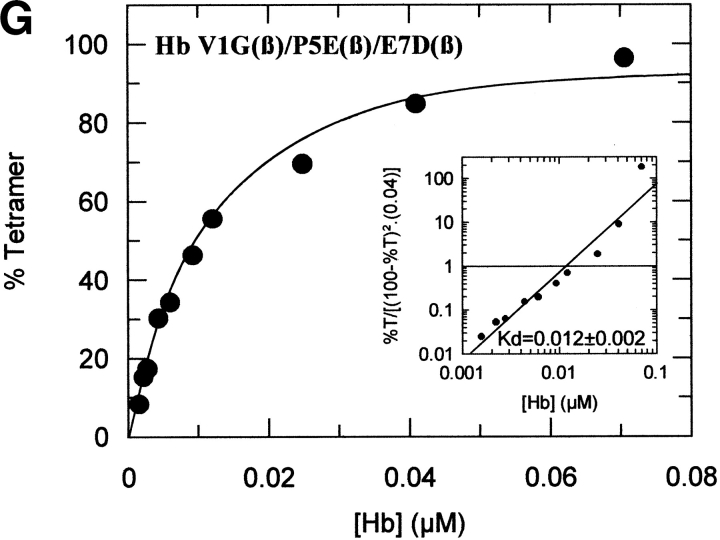
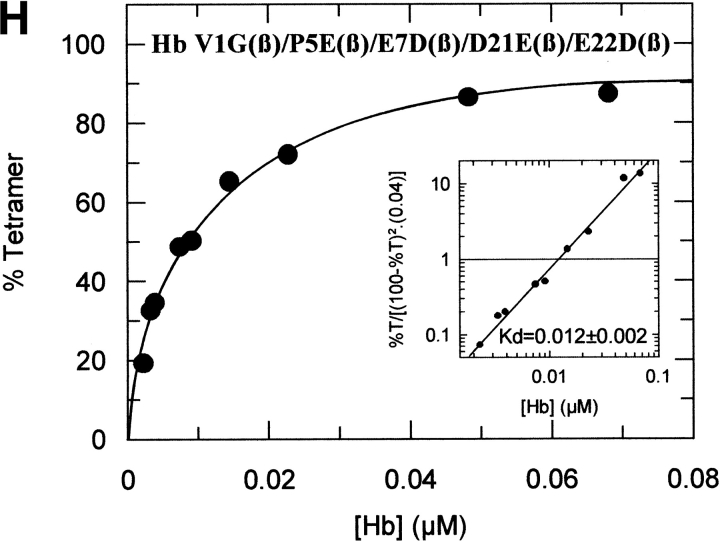
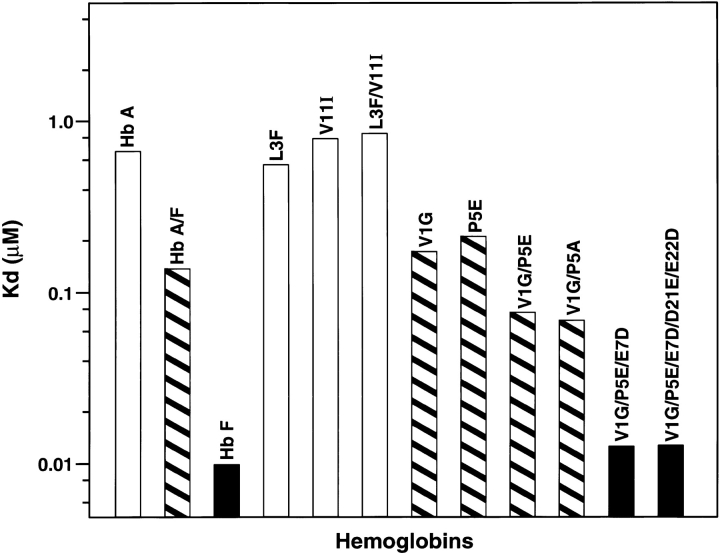
The tetramer-dimer dissociation data for all the mutants are shown in Figure 4 ▶. The hydrophobic substitutions at positions 3 and 11 either alone or in combination have no effect on the Kd values. The effect of the V1G(β) substitution reported previously (Chen et al. 2000) is matched in tetramer strength gain by the P5E(β) substitution, and the combination of both shows some additivity. However, it is not only the presence of Glu 5 that is important as Ala 5 has the same response. Hence, substitution of Pro 5 itself appears to contribute to tetramer strengthening. The triple mutant V1G(β)/P5E(β)/E7D(β) has nearly the same tetramer strength as HbF itself and extending the substitutions to the beginning of the B helix (V1G(β)/P5E(β)/E7D(β)/D21E(β)/E22D(β) does not increase tetramer strength beyond that of HbF itself or that of the triple mutant. A summary of the Kd values for all the recombinant mutants described here as well as some results reported earlier is shown in Figure 5 ▶. The sites on the A helix that have no effect on Kd lowering are shown in the open bars and include the replacements at positions 3 and 11. Intermediate tetramer strength (hatched bars) reported earlier for HbA/F with the five substitutions at the subunit interfaces (Dumoulin et al. 1997) are in the same ranges as those made only at positions 1 and 5. However, when the substitution at position 7 is included in the triple mutant, the tetramer strength is very close to that of HbF (0.01 μM) and of Hb Felix (0.03 μM; Dumoulin et al. 1998). The neutral P5A(β) substitution may involve a change in the helix conformation due to removal of Pro 5. Inspection of the structures shows that the Pro/Ala changes in position 5 could affect sterically Glu 6 and Lys 8. As a result, Lys 8 is 5.57 angstroms from Asp 79 in HbF and 10.85 angstroms from Asp 79 in HbA. This could be because of a Pro 5/Glu 5 steric/structural consequence on Lys 8 directly, or through Glu 6. The inclusion of the more electronegative Asp substitution at position 7 maximizes the effect. Higher order replacements as in the pentuple mutant have no further effect.
Fig. 4.
Tetramer-dimer dissociation constants. The details are described in the text.
Fig. 5.
Summary of tetramer strength (Kd values) for all mutants.
Analysis of electrostatic surface potential
Figure 6 ▶ compares the electrostatic surface potential (GRASP) for the deoxy forms of the β- and γ-subunits of HbA (Fermi et al. 1984) and HbF (Frier and Perutz 1977), respectively. Ribbon diagrams with key residues displayed are provided for orientation and reference. As described above, the overall structural differences between them are not large with the major difference in their A helices. However, the electrostatic surface potential differences are more significant. Because HbA-E7 and HbF-D7 are oriented around the other side of the GRASP views, they are not seen in this orientation. The view centers around the C and FG helices that are involved in the allosteric interface where the Kd measurements are made. The electrostatic surface potential qualitatively supports the stronger interaction between subunits in HbF that has the stronger electronegative region around residues E90/D94. HbF also has the stronger electropositive region around residue R40 and stronger electronegative area around residue D43. Residues E5 and D7 in the A helix of HbF are responsible for the stronger electronegative region compared with P5 and E7 in HbA, which would affect reorientation of side chains and their dipoles that could propagate along the surface thus affecting and enhancing the electronegative FG helix region resulting in a stronger attractive force and a tighter interface. Electrostatic effects are also suggested by the findings (1) that naturally occurring acetylated HbF (Ac-Gly 1[γ]) with its N-terminal charge blocked, behaves like HbA in its tetramer-dimer dissociation properties and (2) that HbF itself at pH 9.0, where Gly 1(γ) is mainly unprotonated, also behaves like HbA; at pH values below 8.0, the parallel dissociation of both HbF and HbA begins and continues as the pH is lowered because of the uptake of protons at an unknown site(s) (Manning and Manning 2001). These results suggest that it is not only the sequence of amino acids of the A helix per se that confers tetramer strength but rather the extent to which the amino acids in the A helix of either the β or γ subunits can assume interacting electropositivity or electronegativity. This conclusion is consistent with the GRASP electrostatic potential difference. Another contributing factor is probably steric because substitution of Pro 5(β) by Ala also contributes to tetramer strength. These considerations indicate that a thorough study of A-helix contributions to subunit contacts is warranted.
Fig. 6.
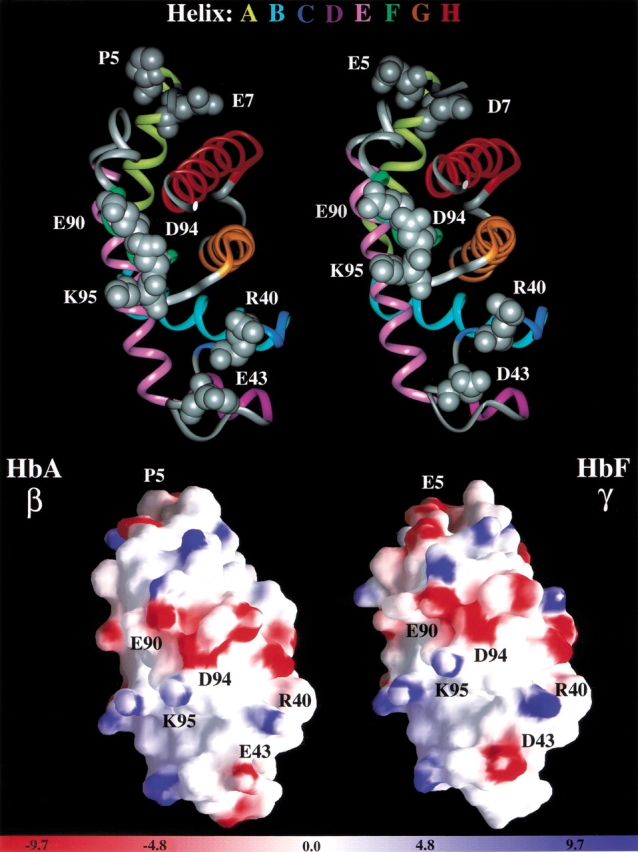
Electrostatic surface potential of β- and γ-subunits of deoxy HbA and deoxy HbF. The details are described in the text.
Oxygen affinity of A helix mutants
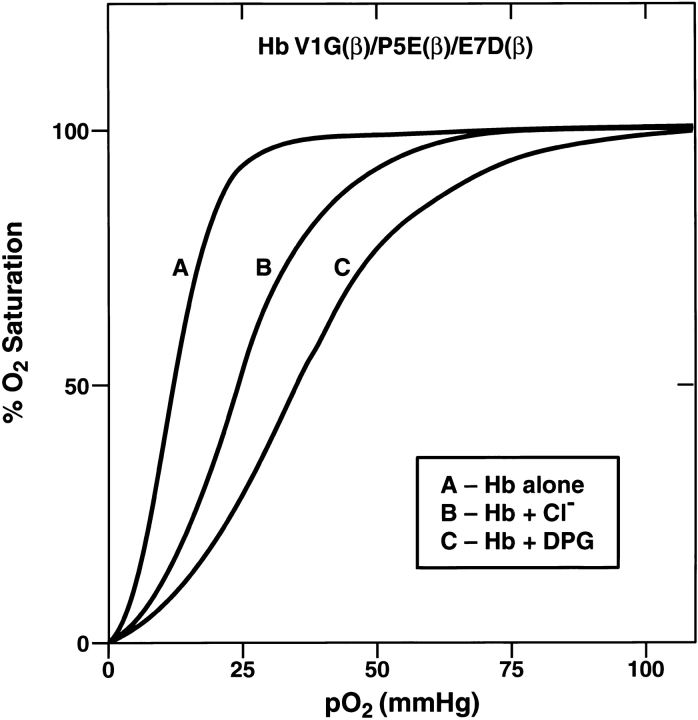
The findings with Hb Felix revealed that its oxygen affinity in the presence or absence of allosteric effectors was the same as that of HbA even though its tetramer-dimer dissociation properties in the R-state were like those of HbF (Dumoulin et al. 1998). On the other hand, HbA/F containing the five-subunit interface amino acids of HbF and the remaining sequence of β-origin was very similar to HbF in its increased oxygen affinity and decreased response to allosteric regulation but had only moderately increased tetramer strength (Dumoulin et al. 1997). Thus, the properties of increased tetramer strength and decreased oxygen affinity that are intrinsic to HbF are separable in Hb Felix and HbA/F. The recombinant mutants described here that participate in tetramer strengthening all have P50 values and DPG or chloride responses close to those of HbA except for L3F(β) and V11I(β), which do not contribute to tetramer strength but have an increased DPG response when present together (Table 2). The triple and the pentuple mutants also have increased DPG responses. A typical oxygen-binding curve for the triple mutant V1G(β)/P5E(β)/E7D(β) is shown in Figure 7 ▶. Analysis for each mutant was performed at least in triplicate with a precision of ±1 mm Hg in P50 values. The data for all the mutants in the absence or presence of allosteric regulators are summarized in Table 2. Studies also were performed with equivalent amounts of inositol hexaphosphate (IHP) to Hb (data not shown); P50 values for all mutants were in the range of 40 to 50 mm Hg in the presence of IHP except for L3F(β), which was 35 mm Hg. The corresponding values for HbA were 57 mm Hg and an n value of 1.7. The results overall emphasize the concept that certain sites on the A helix of the γ-subunit in the R-state translate their effects to the allosteric interface without significantly influencing oxygen binding and release. The single difference between HbF and HbA at this interface, that is, Asp 43(γ) instead of Glu 43(β), must be present to attain such changes (Chen et al. 2000). Recently, Tsai et al. (2001) reported that a converse construct to Hb Felix having an A helix of β amino acids and remaining helices corresponding to γ amino acids had some properties that were not like the ones of HbA that they expected. The reasons for this are not clear. The results in the present report dissect the individual residue contributions of the A helix to the tetramer strengths of Hb Felix and HbF (Fig. 5 ▶).
Table 2.
Summary of P50 and n valuesa
| No additions | +NaCl (200 mM) | +DPG (4 mM) | ||||
| Hemoglobinb | P50 | n | P50 | n | P50 | n |
| HbA | 12 | 2.5 | 22 | 2.9 | 28 | 2.9 |
| HbFc | 7 | 2.7 | 12 | 2.8 | ||
| L3F(β) | 10 | 2.3 | 18 | 1.9 | 31 | 1.6 |
| V11I(β) | 9 | 2.5 | 18 | 2.6 | 25 | 2.6 |
| L3F(β)/V11I(β) | 11 | 2.9 | 22 | 2.4 | 40 | 2.0 |
| P5E(β) | 12 | 2.7 | 19 | 2.7 | 27 | 2.5 |
| V1G(β)/P5E(β) | 12 | 2.7 | 21 | 2.3 | 27 | 2.1 |
| V1G(β)/P5A(β) | 11 | 2.8 | 21 | 2.6 | 31 | 2.5 |
| V1G(β)/P5E(β)/E7D(β) | 11 | 2.8 | 23 | 2.8 | 34 | 2.5 |
| V1G(β)/P5E(β)/E7D(β)/D21E(β)/E22D(β) | 11 | 2.9 | 26 | 2.7 | 38 | 2.6 |
a Average values of three to five determinations.
b The final Hb concentration was 0.8 mM as tetramer for all experiments.
c From Chen et al. 2000.
Fig. 7.
Oxygen binding data for triple mutant. The details are described in the text.
Remote effects in the hemoglobin tetramer
Well-known examples of communication between spatially separated segments of the hemoglobin tetramer are heme–heme interactions responsible for cooperative oxygen binding and the alkaline Bohr effect involving reversible protonation of amino acid side chains to favor a global shift from the R-state to the T-state (Perutz 1989). The remote effects due to the A-helix amino acid sequence shown in Hb Felix on the allosteric tetramer-dimer interface is another such example (Dumoulin et al. 1997,1998). Its mechanism is amenable to study by site-directed mutagenesis as described here. This system also presents the opportunity to study signaling from one part of the tetramer to another. A simple example of this communication is the distant effects of protonation or acetylation at the N terminus of the γ-subunit to influence tightening or relaxing, respectively, of the subunit contacts at the allosteric interface of HbF (Manning and Manning 2001). Such an understanding is important because one physiological role of increased tetramer strength of HbF may be to confer resistance to digestion by malaria proteases (Pasvol et al. 1976), which digest dimers but not tetramers (Shear et al. 1998).
Materials and methods
Mutagenesis and fermentation
The same procedures used for previous site-directed mutagenesis studies (Dumoulin et al. 1997,1998; Martin de Llano et al. 1993,1994) were used here with the following exceptions. Oligonucleotides were prepared by Gene Link. After mutagenesis the entire cDNA sequence for each mutant was confirmed as correct at the Molecular Biology Core Facility of the Forsyth Center in Boston. The yields were improved to 75–150 mg per 15 L fermentation through an initial screening of colonies that showed the best growth on a small scale.
Purification and characterization of hemoglobins
These procedures have been described previously (Dumoulin et al. 1997,1998; Martin de Llano et al. 1993,1994) and provided pure hemoglobins after CM-52 chromatography and Mono S chromatography. The pure hemoglobins were further characterized by mass spectrometry (Beavis and Chait 1996; Li et al. 1999). Circular dichroism measurements were performed on a Jasco 715 as described previously (Martin de Llano and Manning 1994) on liganded Hb in the visible, near ultraviolet and far ultraviolet regions.
Mass spectrometry
The recombinant hemoglobins were characterized using the three-step approach published previously (Li et al. 1999). The average molecular mass of the intact globins were measured using an electrospray-ion trap mass spectrometer, model LCQ-Deca (Finnigan, ThermoQuest) equipped with an in-house constructed electrosprayer. Twenty-nanomolar recombinant hemoglobin solutions in water-methanol-acetic acid (25:74:1, v/v/v) were infused at a constant rate of 1 μL/min−1 through a 50-μm I.D. fused silica capillary into the ion source of the mass spectrometer and electrosprayed at +2.2 kV. Desolvation of protein ions was accomplished by maintaining the heated capillary at 175°C and using a 5% relative collisional energy (Finnigan's nomenclature) at the ion source (source CID). One hundred spectral scans were taken in the mass-to-charge (m/z) range of 600 to 1600 (200-ms injection time and a maximal number of counts of 5 × 108) and averaged before acquisition to produce the final electospray spectrum. The resulting m/z spectrum was deconvoluted using an in-house deconvolution program. The molecular masses of the globins are reported in Table 1.
For proteolysis and sequence analysis, 500 pmoles of recombinant hemoglobin were diluted in 50 mM ammonium bicarbonate, at pH 8.2, and preincubated for 5 min at 37°C. Modified 1-L-tosylamido-2-phenylethyl chloromethyl ketone trypsin (Roche Biochemicals) was added to the reaction and the solution was incubated for 4 h at 37°C with occasional stirring. A second aliquot of trypsin was added after 4 h (final substrate-to-enzyme ratio of 50:1, mole/mole), and the reaction was allowed to proceed for another 4-h period under identical conditions. The final reaction volume was 50 μL with a hemoglobin concentration of 10 μM. The enzymatic hydrolysis was stopped by addition of 50 μL of acetonitrile; aliquots of 1 μL were withdrawn and further diluted 10-fold in water-acetonitrile (1:1 v/v) bringing the peptide concentration of 1 μM. Matrix solutions were prepared by dissolving α-cyano-4-hydroxy-cinnamic acid (4hcca) to its saturation point in either water-acetonitrile (1:1 v/v, 4hcca-WA solution) or water-acetonitrile-TFA (2:1 v/v, 0.1% TFA, 4hcca-TWA solution). The second solution, 4hcca-TWA, was further diluted in water-acetonitrile-TFA (2:1 v/v, 0.1% TFA) to a final 4hcca concentration of 70% of its saturation point. For MALDI-TOF/MS analysis, one-microliter aliquots of the diluted trypsinized hemoglobin solution (1 μM) were rapidly mixed with either 4hcca-WA or 4hcca-TWA, and 0.5-μL aliquots were transferred onto a gold-coated sample plate and allowed to air-dry. The dried spots were washed with cold water (for 4hcca-WA) or cold 0.1% aqueous TFA (for 4hcca-TWA) before analysis in positive and/or negative ion mode on a MALDI-TOF/MS instrument (model Voyager DE-STR, PerSeptive, Perkin Elmer) equipped with delayed extraction and ion reflection.
From the peptide map obtained by MALDI-TOF/MS, peptides expected to contain amino acid substitutions were selected for mass spectrometric fragmentation in an electrospray-ion trap mass spectrometer, model LCQ-Deca. Trypsinized hemoglobin solutions were prepared in either water-methanol-acetic acid (49:50:1 v/v/v, for analysis using positive ionization) or 5 mM ammonium bicarbonate in water-methanol (1:1 v/v, for negative ionization) to a final concentration of 10 nM. The solutions were infused at 1 μL/min−1 through a 50-μm I.D. fused silica capillary into the ion source of the mass spectrometer and electrosprayed at +2.2 kV. Peptide ions were desolvated at a heated capillary temperature of 150°C. Two hundred scans were averaged in profile mode at injection times of 500 ms and maximal number of counts of 5 × 109 to produce a single fragmentation spectrum for each precursor ion selected.
Tetramer-dimer dissociation constants (Kd)
These procedures have been described previously (Manning et al. 1996,1999). The concentration of each Hb was accurately determined by amino acid analysis for calculation of the final Kd value.
Oxygen affinity measurements
These determinations were performed at 37°C at a Hb concentration of 0.8 mM as tetramer as described previously (Dumoulin et al. 1998; Chen et al. 2000) either in the absence or in the presence of 2,3-DPG, IHP, or sodium chloride at concentrations described in the text.
Acknowledgments
We thank Lois R. Manning for advice in determining the tetramer-dimer dissociation constants and Roger Avelino for his help typing the article.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.30602.
References
- Beavis, R.C. and Chait, B.T. 1996. Matrix-assisted laser desorption ionization mass-spectrometry of proteins. Methods Enzymol. 270 519–551. [DOI] [PubMed] [Google Scholar]
- Chen, W., Dumoulin, A., Li, X., Padovan, J.C., Chait, B.T., Buonopane, R., Platt, O.S., Manning, L.R., and Manning, J.M. 2000. Transposing sequences between fetal and adult hemoglobins indicates which subunits and regulatory molecule interfaces are functionally related. Biochemistry 39 3774–3781. [DOI] [PubMed] [Google Scholar]
- Dumoulin, A., Manning, L.R., Jenkins, W.T., Winslow, R.M., and Manning, J.M. 1997. Exchange of subunit interfaces between recombinant adult and fetal hemoglobins. Evidence for a functional inter-relationship among regions of the tetramer. J. Biol. Chem. 272 31326–31332. [DOI] [PubMed] [Google Scholar]
- Dumoulin, A., Padovan, J.C., Manning, L.R., Popowicz, A., Winslow, R.M., Chait, B.T., and Manning, J.M. 1998. The N-terminal sequence affects distant helix interactions in hemoglobin. Implications for mutant proteins from studies on recombinant hemoglobin Felix. J. Biol. Chem. 273 35032–35038. [DOI] [PubMed] [Google Scholar]
- Fermi, G., Perutz, M.F., Shaanan, B., and Fourme, R. 1984. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J. Mol. Biol. 175 159–174. [DOI] [PubMed] [Google Scholar]
- Frier, J.A. and Perutz, M.F. 1977. Structure of human fetal deoxyhaemoglobin. J. Mol. Biol. 112 97–112. [DOI] [PubMed] [Google Scholar]
- Li, X., Himanen, J.-P., Martin de Llano, J.J., Padovan, J.C., Chait, B.T., and Manning, J.M. 1999. Mutational analysis of sickle haemoglobin (Hb) gelation. Biotechnol. Appl. Biochem. 29 165–184. [PubMed] [Google Scholar]
- Manning, J.M. 1981. Preparation of hemoglobin carbamylated at specific NH2-terminal residues. Methods Enzymol. 76 159–167. [DOI] [PubMed] [Google Scholar]
- Manning, L.R. and Manning, J.M. 2001. The acetylation state of human fetal hemoglobin modulates the strength of its subunit interactions: Long-range effects and implications for histone interactions in the nucleosome. Biochemistry 40 1635–1639. [DOI] [PubMed] [Google Scholar]
- Manning, L.R., Jenkins, W.T., Hess, J.R., Vandegriff, K., Winslow, R.M., and Manning, J.M. 1996. Subunit dissociations in natural and recombinant hemoglobins. Protein Sci. 5 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, L.R., Dumoulin, A., Jenkins, W.T., Winslow, R.M., and Manning, J.M. 1999. Determining subunit dissociation constants in natural and recombinant proteins. Methods Enzymol. 306 113–129. [DOI] [PubMed] [Google Scholar]
- Martin de Llano, J.J. and Manning, J.M. 1994. Properties of a recombinant human hemoglobin double mutant: Sickle hemoglobin with Leu-88(β) at the primary aggregation site substituted by Ala. Protein Sci. 3 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin de Llano, J.J., Schneewind, O., Stetler, G., and Manning, J.M. 1993. Recombinant human sickle hemoglobin expressed in yeast. Proc. Natl. Acad. Sci. 90 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin de Llano, J.J., Schneewind, O., Stetler, G.L., and Manning, J.M. 1994. Purification and characterization of recombinant human sickle hemoglobin expressed in yeast. Methods Enzymol. 231 390–403. [DOI] [PubMed] [Google Scholar]
- Pasvol, G., Weatherall, D.J., Wilson, R.J., Smith, D.H., and Gilles, H.M. 1976. Fetal haemoglobin and malaria. Lancet 1269–1272. [DOI] [PubMed]
- Perutz, M. 1989. Mechanisms of cooperativity and allosteric regulation in proteins. Q. Rev. Biophys. 22 139–237. [DOI] [PubMed] [Google Scholar]
- Shear, H.L., Grinberg, J., Gilman, M.E., Fabry, G., Stamatoyannopoulos, G., Goldberg, D.E., and Nagel, R.L. 1998. Transgenic mice expressing human fetal globin are protected from malaria by a novel mechanism. Blood 92 2520–2526. [PubMed] [Google Scholar]
- Tsai, C.-H., Larson, S.C., Shen, T.-J., Ho, N.T., Fisher, G.W., Tam, M.F., and Ho, C. 2001. Probing the importance of the amino-terminal sequence of the β- and γ-chains to the properties of normal adult and fetal hemoglobins. Biochemistry 40 12169–12177. [DOI] [PubMed] [Google Scholar]