Abstract
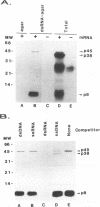
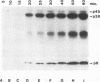
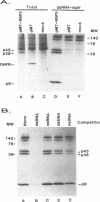
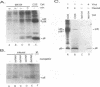
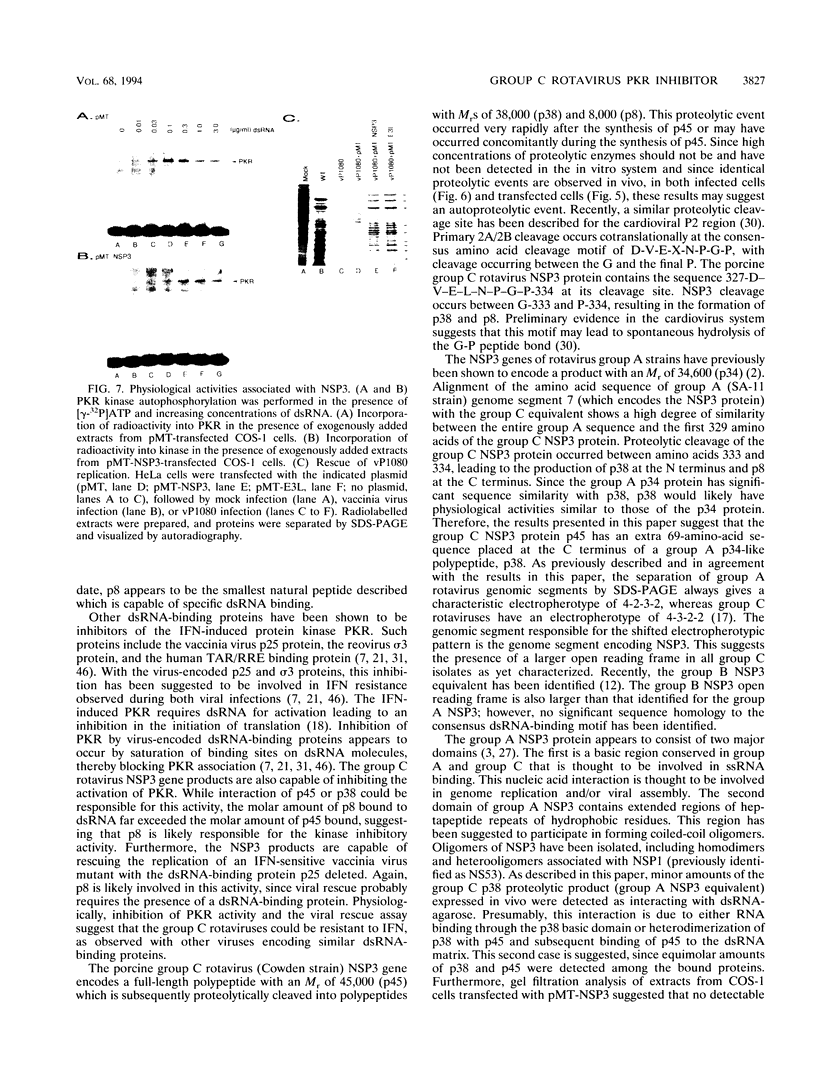
The porcine group C rotavirus (Cowden strain) NSP3 protein (the group C equivalent of the group A gene 7 product, formerly called NS34) shares homology with known double-stranded RNA-binding proteins, such as the interferon-induced, double-stranded RNA-dependent protein kinase PKR. A clone of NSP3, expressed both in vitro and in COS-1 cells, led to the synthesis of minor amounts of a product with an M(r) of 45,000 (the expected full-length M(r) of NSP3) and major amounts of products with M(r)s of 38,000 and 8,000. Restriction enzyme digestion analysis prior to expression in vitro and amino-terminal sequence analysis suggest that the products with M(r)s of 38,000 and 8,000 are cleavage products of the protein with an M(r) of 45,000. The full-length protein and the product with an M(r) of 8,000, both of which contain the motif present in double-stranded RNA-binding proteins, bound specifically to double-stranded RNA. The products with M(r)s of 45,000 and 8,000 were also detected in Cowden strain-infected MA104 cells. NSP3 products expressed in COS-1 cells were capable of inhibiting activation of the double-stranded RNA-dependent protein kinase similar to other double-stranded RNA-binding proteins, and NSP3 products expressed in HeLa cells were capable of rescuing the replication of an interferon-sensitive deletion mutant of vaccinia virus.
Full text
PDF


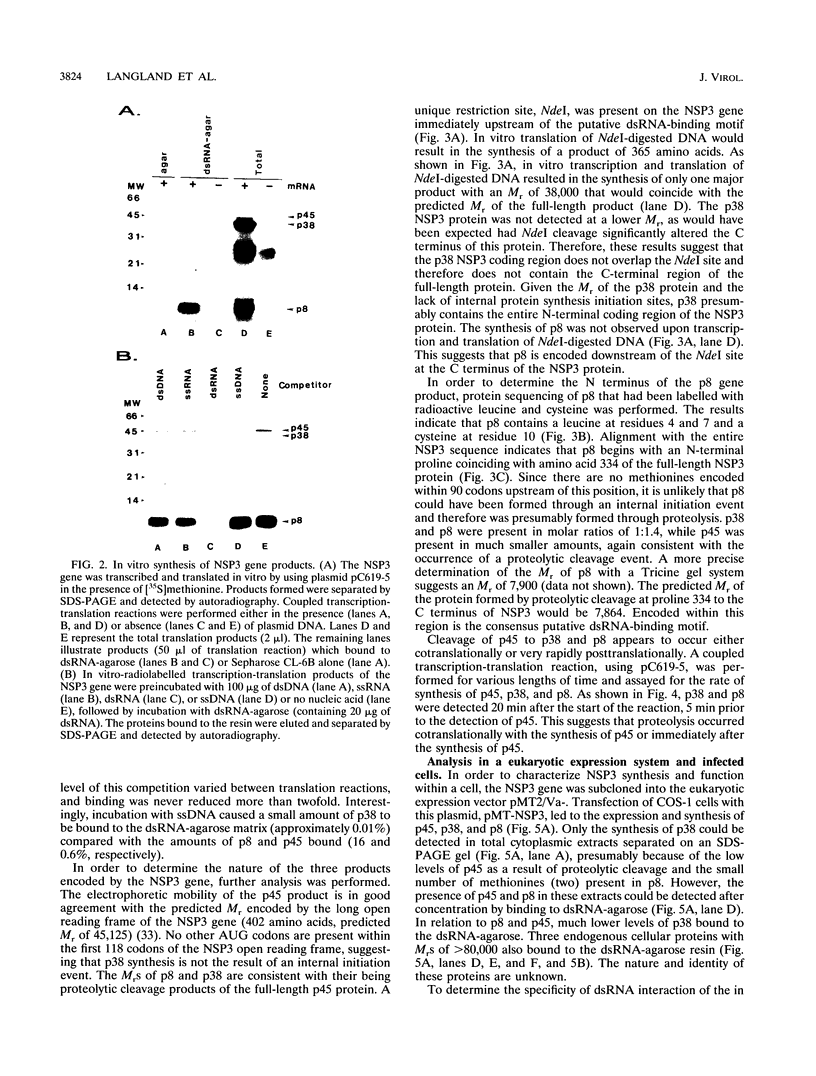


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohl E. H., Saif L. J., Theil K. W., Agnes A. G., Cross R. F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982 Feb;15(2):312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Bellamy A. R., Siegman L. J. Nucleotide sequence of the dsRNA genomic segment 7 of Simian 11 rotavirus. Nucleic Acids Res. 1984 Feb 10;12(3):1621–1626. doi: 10.1093/nar/12.3.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Holmes K. V. RNA-binding proteins of bovine rotavirus. J Virol. 1986 May;58(2):561–568. doi: 10.1128/jvi.58.2.561-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C. Novel rotaviruses in animals and man. Ciba Found Symp. 1987;128:5–23. doi: 10.1002/9780470513460.ch2. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Pedley S., McCrae M. A. Group C rotaviruses in humans. J Clin Microbiol. 1986 Apr;23(4):760–763. doi: 10.1128/jcm.23.4.760-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. W., Watson J. C., Jacobs B. L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Hung T., Bridger J. C., McCrae M. A. Chinese adult rotavirus is a group B rotavirus. Lancet. 1985 Nov 16;2(8464):1123–1124. doi: 10.1016/s0140-6736(85)90710-x. [DOI] [PubMed] [Google Scholar]
- Christensen M. L. Human viral gastroenteritis. Clin Microbiol Rev. 1989 Jan;2(1):51–89. doi: 10.1128/cmr.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu N. M., Janckila A. J., Wallace J. H., Yam L. T. Assessment of a method for immunochemical detection of antigen on nitrocellulose membranes. J Histochem Cytochem. 1989 Feb;37(2):257–263. doi: 10.1177/37.2.2536057. [DOI] [PubMed] [Google Scholar]
- Edelman R. Perspective on the development and deployment of rotavirus vaccines. Pediatr Infect Dis J. 1987 Aug;6(8):704–710. doi: 10.1097/00006454-198708000-00002. [DOI] [PubMed] [Google Scholar]
- Eiden J. J. Gene 5 of the IDIR agent (group B rotavirus) encodes a protein equivalent to NS34 of group A rotavirus. Virology. 1993 Sep;196(1):298–302. doi: 10.1006/viro.1993.1479. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Giantini M., Shatkin A. J. Stimulation of chloramphenicol acetyltransferase mRNA translation by reovirus capsid polypeptide sigma 3 in cotransfected COS cells. J Virol. 1989 Jun;63(6):2415–2421. doi: 10.1128/jvi.63.6.2415-2421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989 Dec;9(6):641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- Hrdy D. B. Epidemiology of rotaviral infection in adults. Rev Infect Dis. 1987 May-Jun;9(3):461–469. doi: 10.1093/clinids/9.3.461. [DOI] [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Yao H. L., Fang Z. Y., Chao T. X., Chou Z. Y., Ye W., Chang X. J., Den S. S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984 May 26;1(8387):1139–1142. [PubMed] [Google Scholar]
- Imani F., Jacobs B. L. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7887–7891. doi: 10.1073/pnas.85.21.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B. M., Saif L. J., Kang S. Y., Kim J. H. Biochemical characterization of the structural and nonstructural polypeptides of a porcine group C rotavirus. J Virol. 1990 Jul;64(7):3171–3178. doi: 10.1128/jvi.64.7.3171-3178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell A. K., Poruchynsky M. S., Bellamy A. R., Greenberg H. B., Atkinson P. H. Two forms of VP7 are involved in assembly of SA11 rotavirus in endoplasmic reticulum. J Virol. 1988 Aug;62(8):2929–2941. doi: 10.1128/jvi.62.8.2929-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Stark G. R. The antiviral effects of the interferons and their inhibition. J Interferon Res. 1992 Aug;12(4):237–240. doi: 10.1089/jir.1992.12.237. [DOI] [PubMed] [Google Scholar]
- Langland J. O., Jacobs B. L. Cytosolic double-stranded RNA-dependent protein kinase is likely a dimer of partially phosphorylated Mr = 66,000 subunits. J Biol Chem. 1992 May 25;267(15):10729–10736. [PubMed] [Google Scholar]
- Mattion N. M., Cohen J., Aponte C., Estes M. K. Characterization of an oligomerization domain and RNA-binding properties on rotavirus nonstructural protein NS34. Virology. 1992 Sep;190(1):68–83. doi: 10.1016/0042-6822(92)91193-x. [DOI] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Wang S. S., Gary G. W., Melnick J. L. Detection of antibody to group B adult diarrhea rotaviruses in humans. J Clin Microbiol. 1987 May;25(5):812–818. doi: 10.1128/jcm.25.5.812-818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I., Yamazaki K., Minekawa Y. An occurrence of diarrheal cases associated with group C rotavirus in adults. Microbiol Immunol. 1993;37(6):505–509. doi: 10.1111/j.1348-0421.1993.tb03243.x. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C., Parks G. D., Hall D. J., Ingraham R. H., Seng T. W., Pallai P. V. Proteolytic processing of the cardioviral P2 region: primary 2A/2B cleavage in clone-derived precursors. Virology. 1992 Oct;190(2):754–762. doi: 10.1016/0042-6822(92)90913-a. [DOI] [PubMed] [Google Scholar]
- Poncet D., Aponte C., Cohen J. Rotavirus protein NSP3 (NS34) is bound to the 3' end consensus sequence of viral mRNAs in infected cells. J Virol. 1993 Jun;67(6):3159–3165. doi: 10.1128/jvi.67.6.3159-3165.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y. A., Jiang B. M., Saif L. J., Kang S. Y., Ojeh C. K., Green K. Y. Molecular analysis of the gene 6 from a porcine group C rotavirus that encodes the NS34 equivalent of group A rotaviruses. Virology. 1991 Oct;184(2):752–757. doi: 10.1016/0042-6822(91)90446-i. [DOI] [PubMed] [Google Scholar]
- Saif L. J., Terrett L. A., Miller K. L., Cross R. F. Serial propagation of porcine group C rotavirus (pararotavirus) in a continuous cell line and characterization of the passaged virus. J Clin Microbiol. 1988 Jul;26(7):1277–1282. doi: 10.1128/jcm.26.7.1277-1282.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991 Jul;183(1):1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Lengyel P. The interferon system. A bird's eye view of its biochemistry. J Biol Chem. 1992 Mar 15;267(8):5017–5020. [PubMed] [Google Scholar]
- Snodgrass D. R., Herring A. J., Campbell I., Inglis J. M., Hargreaves F. D. Comparison of atypical rotaviruses from calves, piglets, lambs and man. J Gen Virol. 1984 May;65(Pt 5):909–914. doi: 10.1099/0022-1317-65-5-909. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Brown N. H., Gall J. G., Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. Q., Wu Y. L., Shen H. K., Wang D. B., Chen Y. H., Wu D. M., He L. N., Yang Z. L. An outbreak of epidemic diarrhoea in adults caused by a new rotavirus in Anhui Province of China in the summer of 1983. J Med Virol. 1986 Jun;19(2):167–173. doi: 10.1002/jmv.1890190210. [DOI] [PubMed] [Google Scholar]
- Tao H. Rotavirus and adult diarrhea. Adv Virus Res. 1988;35:193–218. [PubMed] [Google Scholar]
- Tsunemitsu H., Saif L. J., Jiang B. M., Shimizu M., Hiro M., Yamaguchi H., Ishiyama T., Hirai T. Isolation, characterization, and serial propagation of a bovine group C rotavirus in a monkey kidney cell line (MA104). J Clin Microbiol. 1991 Nov;29(11):2609–2613. doi: 10.1128/jcm.29.11.2609-2613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima H., Honma H., Mukoyama A., Shinozaki T., Fujita Y., Kobayashi M., Ohseto M., Morikawa S., Kitamura T. Detection of group C rotaviruses in Tokyo. J Med Virol. 1989 Apr;27(4):299–303. doi: 10.1002/jmv.1890270408. [DOI] [PubMed] [Google Scholar]
- Watson J. C., Chang H. W., Jacobs B. L. Characterization of a vaccinia virus-encoded double-stranded RNA-binding protein that may be involved in inhibition of the double-stranded RNA-dependent protein kinase. Virology. 1991 Nov;185(1):206–216. doi: 10.1016/0042-6822(91)90768-7. [DOI] [PubMed] [Google Scholar]