Abstract
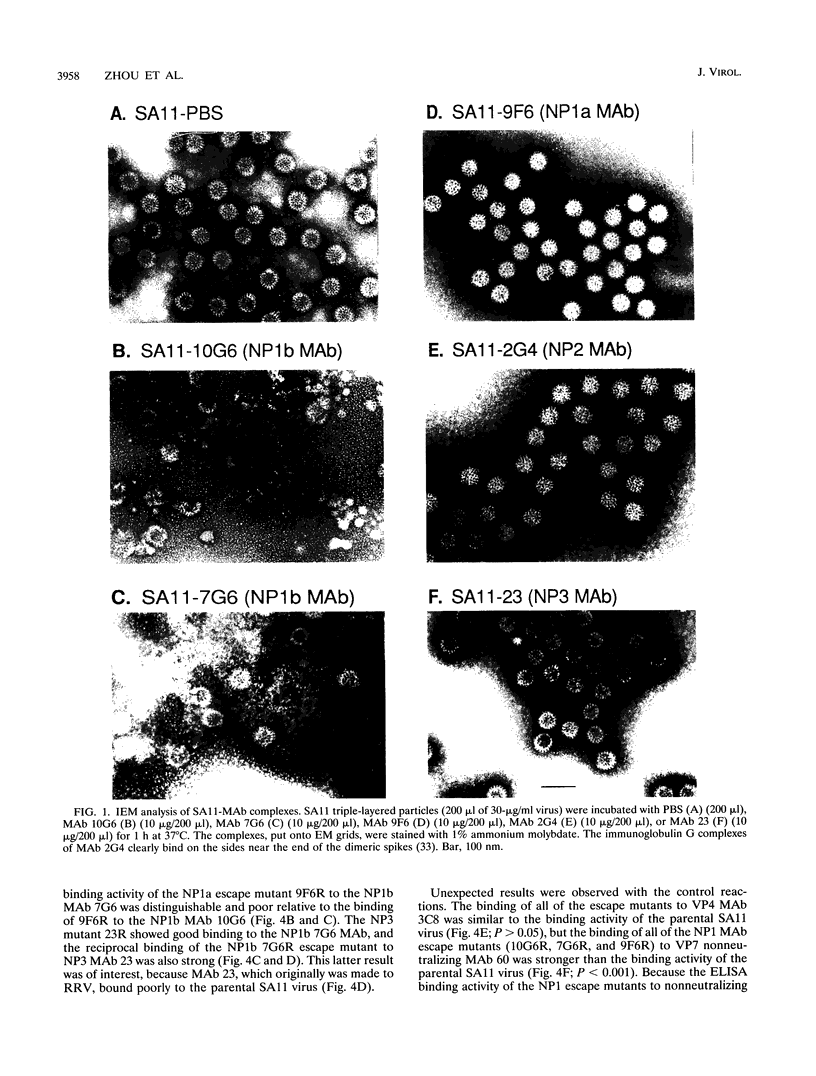
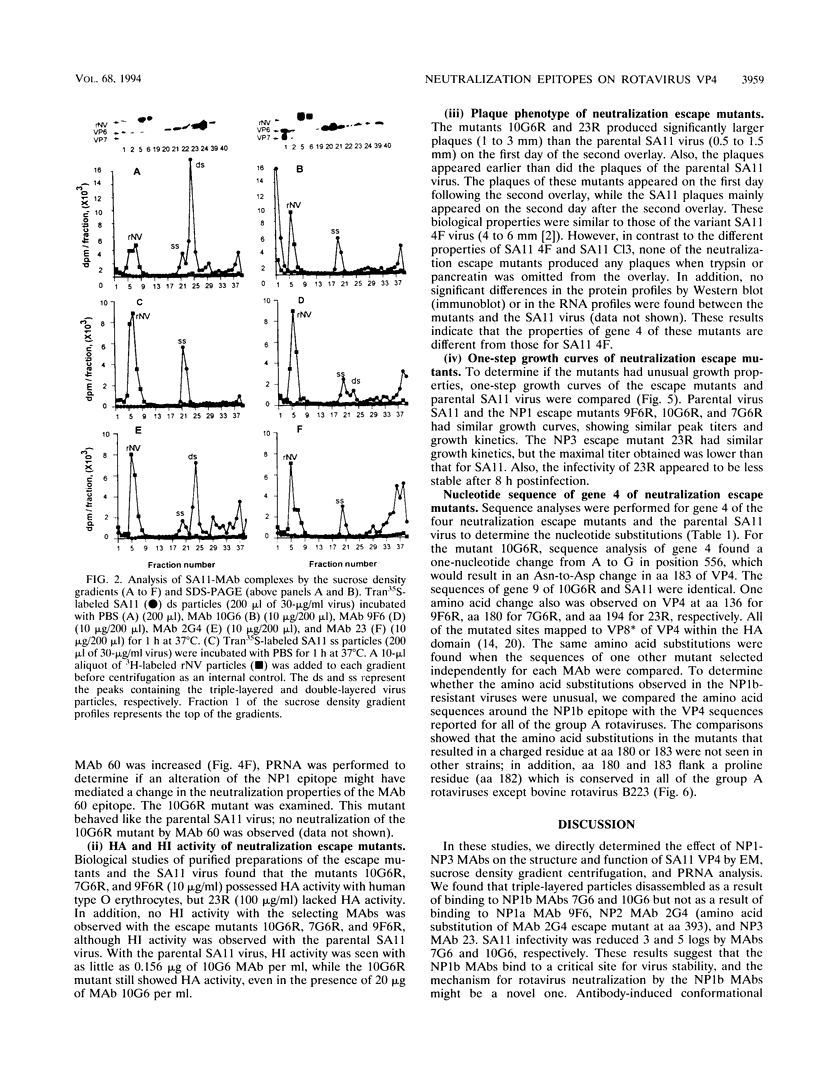
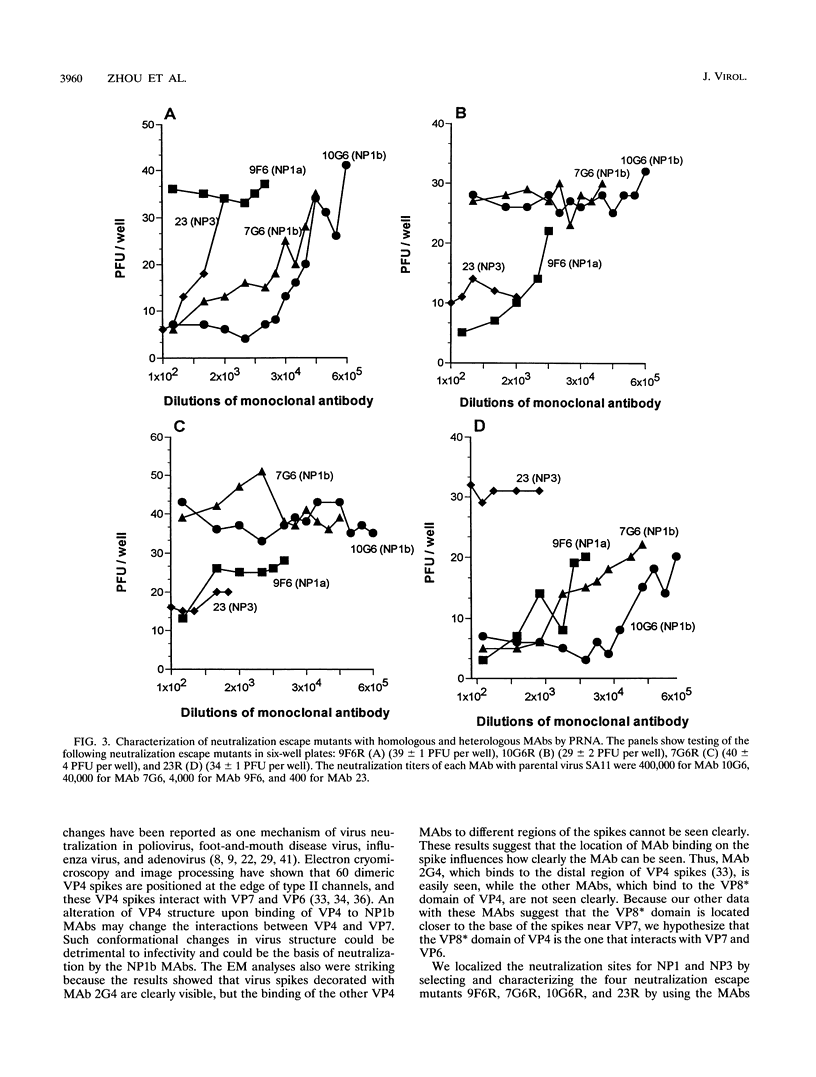
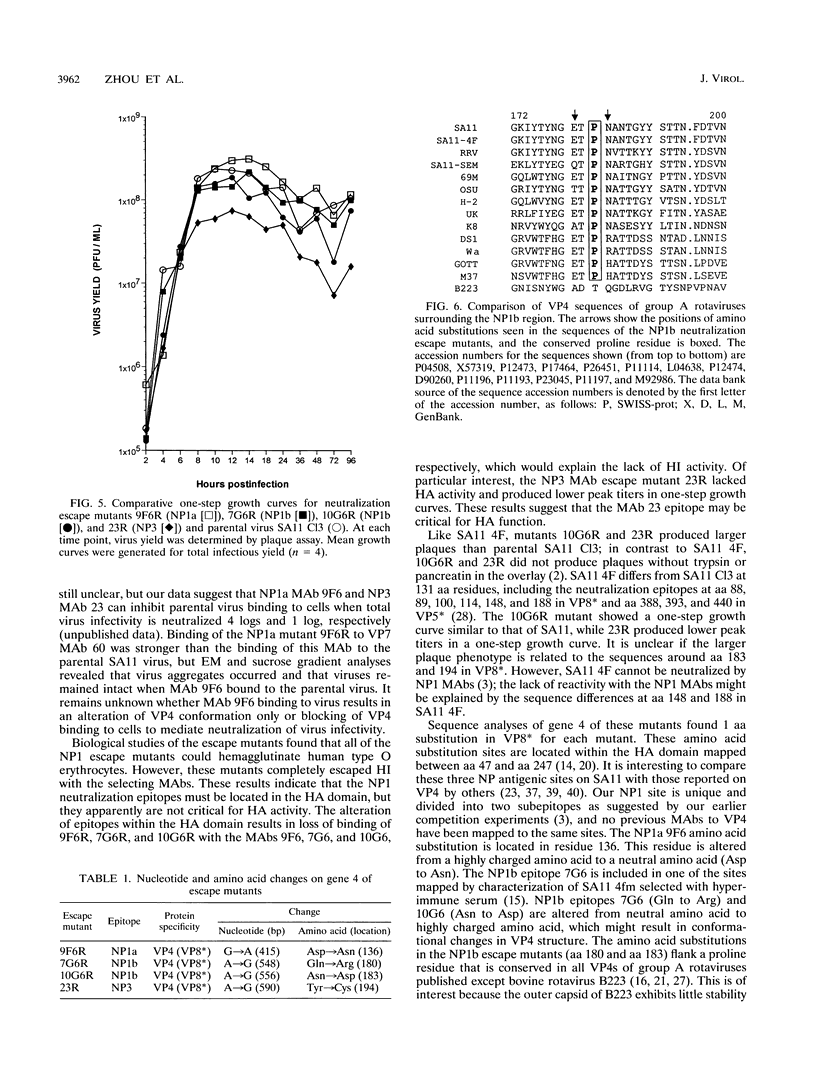
We previously characterized three neutralization-positive epitopes (NP1 [1a and 1b], NP2, and NP3) and three neutralization-negative epitopes on the simian rotavirus SA11 VP4 with 13 monoclonal antibodies (MAbs). Conformational changes occurred as a result of the binding of NP1 MAbs to the SA11 spike VP4, and enhanced binding of all neutralization-negative MAbs was observed when NP1 MAbs bound VP4 in a competitive MAb capture enzyme-linked immunosorbent assay. To further understand the structure and function of VP4, we have continued studies with these MAbs. Electron microscopic and sucrose gradient analyses of SA11-MAb complexes showed that triple-layered viral particles disassembled following treatment with NP1b MAbs 10G6 and 7G6 but not following treatment with NP1a MAb 9F6, NP2 MAb 2G4, and NP3 MAb 23. Virus infectivity was reduced approximately 3 to 5 logs by the NP1b MAbs. These results suggest that NP1b MAb neutralization occurs by a novel mechanism. We selected four neutralization escape mutants of SA11 with these VP4 MAbs and characterized them by using plaque reduction neutralization assays, hemagglutination inhibition assays, and an antigen capture enzyme-linked immunosorbent assay. These analyses support the previous assignment of the NP1a, NP1b, NP2, and NP3 MAbs into separate epitopes and confirmed that the viruses were truly neutralization escape mutants. Nucleotide sequence analyses found 1 amino acid (aa) substitution in VP8* of VP4 at (i) aa 136 for NP1a MAb mutant 9F6R, (ii) aa 180 and 183 for NP1b MAb mutants 7G6R and 10G6R, respectively, and (iii) aa 194 for NP3 MAb mutant 23R. The NP1b MAb mutants showed an unexpected enhanced binding with heterologous nonneutralization MAb to VP7 compared with parental SA11 and the other mutants. Taken together, these results suggest that the NP1b epitope is a critical site for VP4 and VP7 interactions and for virus stability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar R. L., Metcalf T. G., Neill F. H., Estes M. K. Detection of enteric viruses in oysters by using the polymerase chain reaction. Appl Environ Microbiol. 1993 Feb;59(2):631–635. doi: 10.1128/aem.59.2.631-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. W., Chen D., Estes M. K., Ramig R. F. Biological and immunological characterization of a simian rotavirus SA11 variant with an altered genome segment 4. Virology. 1989 Apr;169(2):427–435. doi: 10.1016/0042-6822(89)90168-2. [DOI] [PubMed] [Google Scholar]
- Burns J. W., Greenberg H. B., Shaw R. D., Estes M. K. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J Virol. 1988 Jun;62(6):2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caust J., Dyall-Smith M. L., Lazdins I., Holmes I. H. Glycosylation, an important modifier of rotavirus antigenicity. Arch Virol. 1987;96(3-4):123–134. doi: 10.1007/BF01320955. [DOI] [PubMed] [Google Scholar]
- Chen D. Y., Estes M. K., Ramig R. F. Specific interactions between rotavirus outer capsid proteins VP4 and VP7 determine expression of a cross-reactive, neutralizing VP4-specific epitope. J Virol. 1992 Jan;66(1):432–439. doi: 10.1128/jvi.66.1.432-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Y., Ramig R. F. Determinants of rotavirus stability and density during CsCl purification. Virology. 1992 Jan;186(1):228–237. doi: 10.1016/0042-6822(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. doi: 10.1099/0022-1317-65-6-1015. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Ostapchuk P., Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. doi: 10.1128/jvi.48.2.547-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Identification of rotaviruses of different origins by the plaque-reduction test. Am J Vet Res. 1980 Jan;41(1):151–152. [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Fiore L., Greenberg H. B., Mackow E. R. The VP8 fragment of VP4 is the rhesus rotavirus hemagglutinin. Virology. 1991 Apr;181(2):553–563. doi: 10.1016/0042-6822(91)90888-i. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Larralde G., Ward R. L. Neutralization epitopes on rotavirus SA11 4fM outer capsid proteins. J Virol. 1990 Sep;64(9):4534–4539. doi: 10.1128/jvi.64.9.4534-4539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. E., Gorziglia M., Woode G. N. Amino acid sequence analysis of bovine rotavirus B223 reveals a unique outer capsid protein VP4 and confirms a third bovine VP4 type. Virology. 1992 Nov;191(1):291–300. doi: 10.1016/0042-6822(92)90191-q. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang M., Graham D. Y., Estes M. K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992 Nov;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizano M., López S., Arias C. F. The amino-terminal half of rotavirus SA114fM VP4 protein contains a hemagglutination domain and primes for neutralizing antibodies to the virus. J Virol. 1991 Mar;65(3):1383–1391. doi: 10.1128/jvi.65.3.1383-1391.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck M. D., Gerhard W. Topological mapping antigenic sites on the influenza A/PR/8/34 virus hemagglutinin using monoclonal antibodies. Virology. 1981 Aug;113(1):64–72. doi: 10.1016/0042-6822(81)90136-7. [DOI] [PubMed] [Google Scholar]
- López S., López I., Romero P., Méndez E., Soberón X., Arias C. F. Rotavirus YM gene 4: analysis of its deduced amino acid sequence and prediction of the secondary structure of the VP4 protein. J Virol. 1991 Jul;65(7):3738–3745. doi: 10.1128/jvi.65.7.3738-3745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. M., Mackow E. R., Greenberg H. B. Molecular determinant of rotavirus neutralization and protection. Adv Virus Res. 1989;36:181–214. doi: 10.1016/s0065-3527(08)60585-0. [DOI] [PubMed] [Google Scholar]
- Matsui S. M., Offit P. A., Vo P. T., Mackow E. R., Benfield D. A., Shaw R. D., Padilla-Noriega L., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J Clin Microbiol. 1989 Apr;27(4):780–782. doi: 10.1128/jcm.27.4.780-782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattion N. M., Estes M. K. Sequence of a rotavirus gene 4 associated with unique biologic properties. Arch Virol. 1991;120(1-2):109–113. doi: 10.1007/BF01310953. [DOI] [PubMed] [Google Scholar]
- McCullough K. C., Smale C. J., Carpenter W. C., Crowther J. R., Brocchi E., De Simone F. Conformational alteration in foot-and-mouth disease virus virion capsid structure after complexing with monospecific antibody. Immunology. 1987 Jan;60(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Ruggeri F. M., Greenberg H. B. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991 May;65(5):2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. L., Rothnagel R., Chen D., Ramig R. F., Chiu W., Prasad B. V. Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell. 1993 Aug 27;74(4):693–701. doi: 10.1016/0092-8674(93)90516-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Hoshino Y., Nishikawa K., Green K. Y., Maloy W. L., Morita Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J Virol. 1988 Jun;62(6):1870–1874. doi: 10.1128/jvi.62.6.1870-1874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Maloy W. L., Nishikawa K., Green K. Y., Hoshino Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J Virol. 1988 Jul;62(7):2421–2426. doi: 10.1128/jvi.62.7.2421-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988 Jul;62(7):2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]