Abstract
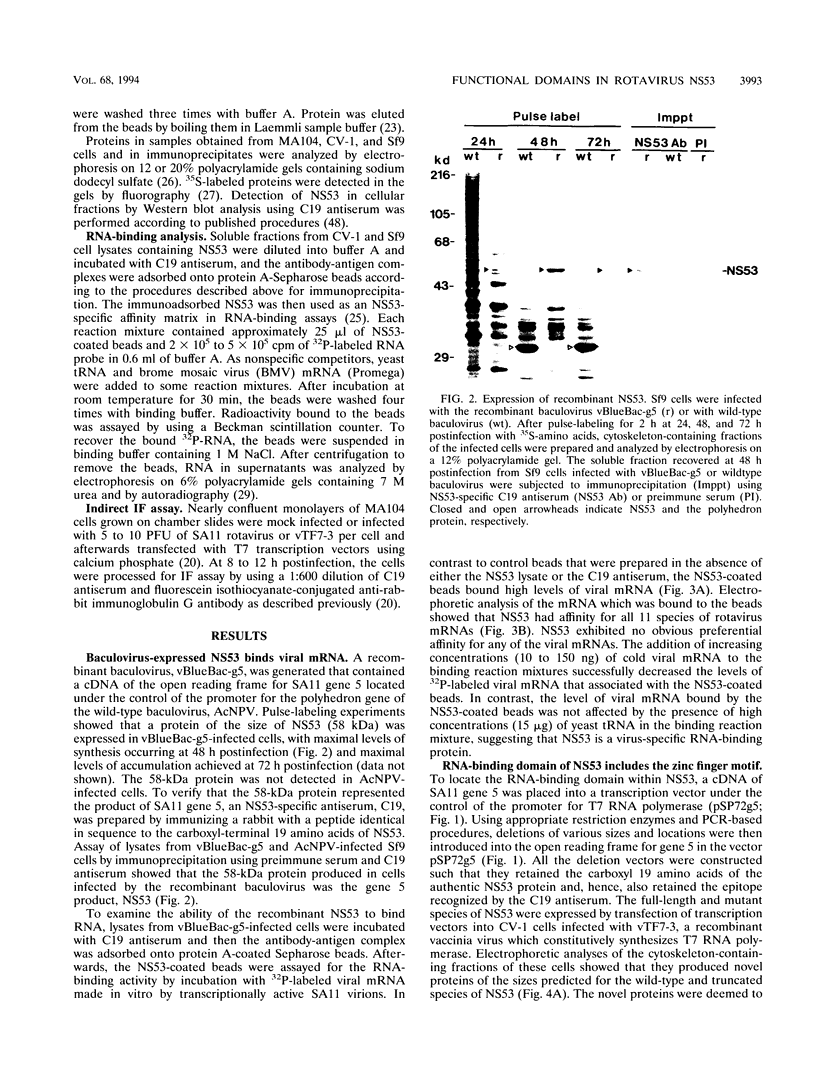
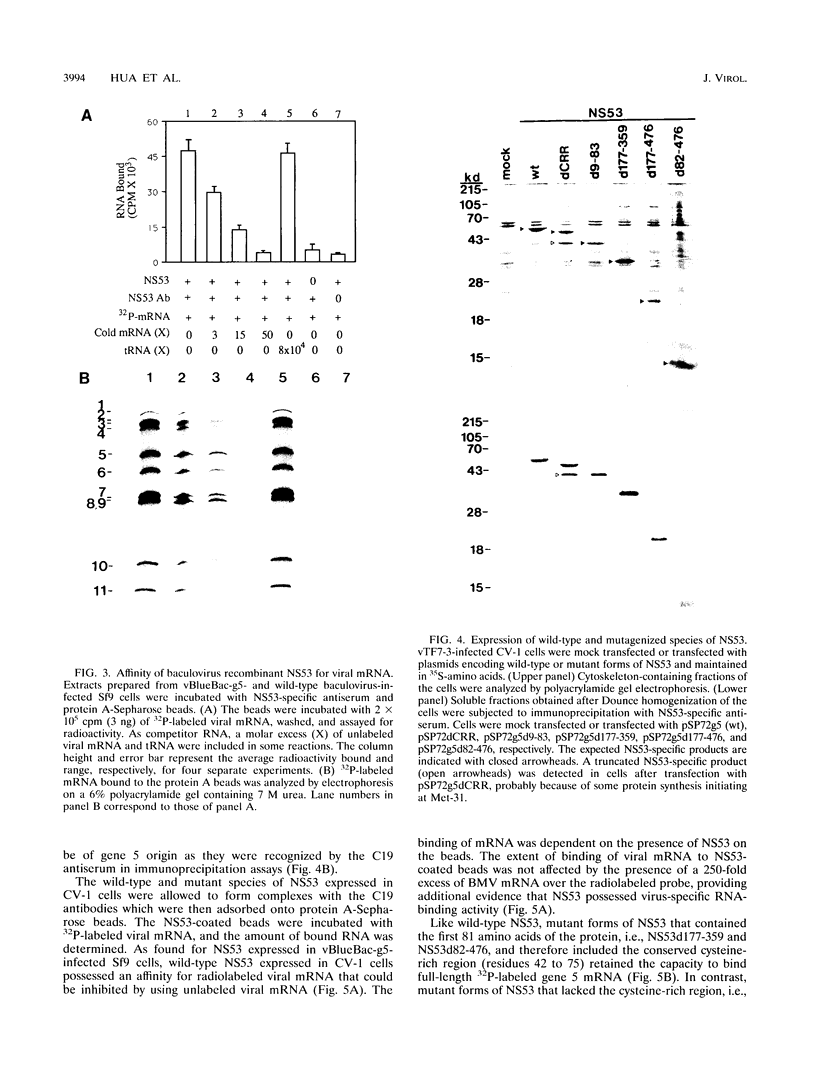
NS53 (NSP1), the gene 5 product of the group A rotaviruses, is a minor nonstructural protein of 486 to 495 amino acids which binds zinc and contains an amino-terminal highly conserved cysteine-rich region that may form one or two zinc fingers. To study the structure-function of the gene 5 product, wild-type and mutant forms of NS53 were produced by using a recombinant baculovirus expression system and a recombinant vaccinia virus/T7 (vTF7-3) expression system. Analysis of the RNA-binding activity of the wild-type NS53 immobilized onto protein A-Sepharose beads with NS53-specific antiserum showed that the protein exhibited specific affinity for all 11 rotavirus mRNAs. The use of short virus-specific RNA probes indicated that NS53 specifically recognizes an element located near the 5' ends of viral mRNAs. Analysis of the RNA-binding activity of deletion mutants of NS53 showed that the RNA-binding domain resides within the first 81 amino acids of the protein and that the highly conserved cysteine-rich region within this region of the protein is essential for the activity. Gel electrophoresis and Western immunoblot analyses of intracellular fractions derived from infected cells revealed that large amounts of NS53 were present in the cytosol and in association with the cytoskeletal matrix. Indirect immunofluorescence analysis of cells programmed to transiently express mutant forms of NS53 using vTF7-3 indicated that the intracellular localization domain resides between amino acids 84 and 176 of NS53. Together, these data show that the RNA-binding domain and the intracellular localization domain lie upstream from the region of NS53 previously determined not to be essential for replication of rotaviruses in cell culture (J. Hua and J. T. Patton, Virology 198:567-576, 1994).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Zinc finger domains: hypotheses and current knowledge. Annu Rev Biophys Biophys Chem. 1990;19:405–421. doi: 10.1146/annurev.bb.19.060190.002201. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Blacklow N. R., Greenberg H. B. Viral gastroenteritis. N Engl J Med. 1991 Jul 25;325(4):252–264. doi: 10.1056/NEJM199107253250406. [DOI] [PubMed] [Google Scholar]
- Bremont M., Chabanne-Vautherot D., Cohen J. Sequence analysis of three non structural proteins of a porcine group C (Cowden strain) rotavirus. Arch Virol. 1993;130(1-2):85–92. doi: 10.1007/BF01318998. [DOI] [PubMed] [Google Scholar]
- Broome R. L., Vo P. T., Ward R. L., Clark H. F., Greenberg H. B. Murine rotavirus genes encoding outer capsid proteins VP4 and VP7 are not major determinants of host range restriction and virulence. J Virol. 1993 May;67(5):2448–2455. doi: 10.1128/jvi.67.5.2448-2455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brottier P., Nandi P., Bremont M., Cohen J. Bovine rotavirus segment 5 protein expressed in the baculovirus system interacts with zinc and RNA. J Gen Virol. 1992 Aug;73(Pt 8):1931–1938. doi: 10.1099/0022-1317-73-8-1931. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Flint S. J. Partition of E1A proteins between soluble and structural fractions of adenovirus-infected and -transformed cells. J Virol. 1986 Dec;60(3):1018–1026. doi: 10.1128/jvi.60.3.1018-1026.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Gombold J. L., Ramig R. F. Intracellular RNA synthesis directed by temperature-sensitive mutants of simian rotavirus SA11. Virology. 1990 Sep;178(1):143–151. doi: 10.1016/0042-6822(90)90387-7. [DOI] [PubMed] [Google Scholar]
- Clapp L. L., Patton J. T. Rotavirus morphogenesis: domains in the major inner capsid protein essential for binding to single-shelled particles and for trimerization. Virology. 1991 Feb;180(2):697–708. doi: 10.1016/0042-6822(91)90083-n. [DOI] [PubMed] [Google Scholar]
- Dalby B., Glover D. M. 3' non-translated sequences in Drosophila cyclin B transcripts direct posterior pole accumulation late in oogenesis and peri-nuclear association in syncytial embryos. Development. 1992 Aug;115(4):989–997. doi: 10.1242/dev.115.4.989. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Wertz G. W. Synthesis of vesicular stomatitis virus negative-strand RNA in vitro: dependence on viral protein synthesis. J Virol. 1982 Mar;41(3):821–832. doi: 10.1128/jvi.41.3.821-832.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont P. S., Hanson I. M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991 Feb 8;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos C. O., Patton J. T. Characterization of rotavirus replication intermediates: a model for the assembly of single-shelled particles. Virology. 1989 Oct;172(2):616–627. doi: 10.1016/0042-6822(89)90204-3. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Estes M. K., Ramig R. F. Assignment of simian rotavirus SA11 temperature-sensitive mutant groups B and E to genome segments. Virology. 1985 May;143(1):309–320. doi: 10.1016/0042-6822(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb E. The 3' untranslated region of localized maternal messages contains a conserved motif involved in mRNA localization. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7164–7168. doi: 10.1073/pnas.89.15.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J., Mansell E. A., Patton J. T. Comparative analysis of the rotavirus NS53 gene: conservation of basic and cysteine-rich regions in the protein and possible stem-loop structures in the RNA. Virology. 1993 Sep;196(1):372–378. doi: 10.1006/viro.1993.1492. [DOI] [PubMed] [Google Scholar]
- Hua J., Patton J. T. The carboxyl-half of the rotavirus nonstructural protein NS53 (NSP1) is not required for virus replication. Virology. 1994 Feb;198(2):567–576. doi: 10.1006/viro.1994.1068. [DOI] [PubMed] [Google Scholar]
- Hundley F., Biryahwaho B., Gow M., Desselberger U. Genome rearrangements of bovine rotavirus after serial passage at high multiplicity of infection. Virology. 1985 May;143(1):88–103. doi: 10.1016/0042-6822(85)90099-6. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Kattoura M. D., Clapp L. L., Patton J. T. The rotavirus nonstructural protein, NS35, possesses RNA-binding activity in vitro and in vivo. Virology. 1992 Dec;191(2):698–708. doi: 10.1016/0042-6822(92)90245-k. [DOI] [PubMed] [Google Scholar]
- Köster M., Kühn U., Bouwmeester T., Nietfeld W., el-Baradi T., Knöchel W., Pieler T. Structure, expression and in vitro functional characterization of a novel RNA binding zinc finger protein from Xenopus. EMBO J. 1991 Oct;10(10):3087–3093. doi: 10.1002/j.1460-2075.1991.tb07861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mansell E. A., Patton J. T. Rotavirus RNA replication: VP2, but not VP6, is necessary for viral replicase activity. J Virol. 1990 Oct;64(10):4988–4996. doi: 10.1128/jvi.64.10.4988-4996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattion N. M., Cohen J., Aponte C., Estes M. K. Characterization of an oligomerization domain and RNA-binding properties on rotavirus nonstructural protein NS34. Virology. 1992 Sep;190(1):68–83. doi: 10.1016/0042-6822(92)91193-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. B., Both G. W. Conservation of a potential metal binding motif despite extensive sequence diversity in the rotavirus nonstructural protein NS53. Virology. 1990 Feb;174(2):618–621. doi: 10.1016/0042-6822(90)90117-a. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg F. S., Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993 Oct 8;75(1):165–174. [PubMed] [Google Scholar]
- Pattnaik A. K., Ball L. A., LeGrone A. W., Wertz G. W. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992 Jun 12;69(6):1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Gallegos C. O. Structure and protein composition of the rotavirus replicase particle. Virology. 1988 Oct;166(2):358–365. doi: 10.1016/0042-6822(88)90506-5. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Salter-Cid L., Kalbach A., Mansell E. A., Kattoura M. Nucleotide and amino acid sequence analysis of the rotavirus nonstructural RNA-binding protein NS35. Virology. 1993 Feb;192(2):438–446. doi: 10.1006/viro.1993.1059. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Greenberg H. B., Graham D. Y., Estes M. K. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1984;1(2):133–152. doi: 10.1016/0168-1702(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Poncet D., Aponte C., Cohen J. Rotavirus protein NSP3 (NS34) is bound to the 3' end consensus sequence of viral mRNAs in infected cells. J Virol. 1993 Jun;67(6):3159–3165. doi: 10.1128/jvi.67.6.3159-3165.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Petrie B. L. Characterization of temperature-sensitive mutants of simian rotavirus SA11: protein synthesis and morphogenesis. J Virol. 1984 Mar;49(3):665–673. doi: 10.1128/jvi.49.3.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Beuchle D., Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991 Jul 12;66(1):51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- Theunissen O., Rudt F., Guddat U., Mentzel H., Pieler T. RNA and DNA binding zinc fingers in Xenopus TFIIIA. Cell. 1992 Nov 13;71(4):679–690. doi: 10.1016/0092-8674(92)90601-8. [DOI] [PubMed] [Google Scholar]
- Tian Y., Tarlow O., Ballard A., Desselberger U., McCrae M. A. Genomic concatemerization/deletion in rotaviruses: a new mechanism for generating rapid genetic change of potential epidemiological importance. J Virol. 1993 Nov;67(11):6625–6632. doi: 10.1128/jvi.67.11.6625-6632.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Vialard J., Lalumière M., Vernet T., Briedis D., Alkhatib G., Henning D., Levin D., Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990 Jan;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisraeli J. K., Sokol S., Melton D. A. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990 Feb;108(2):289–298. doi: 10.1242/dev.108.2.289. [DOI] [PubMed] [Google Scholar]








