Abstract
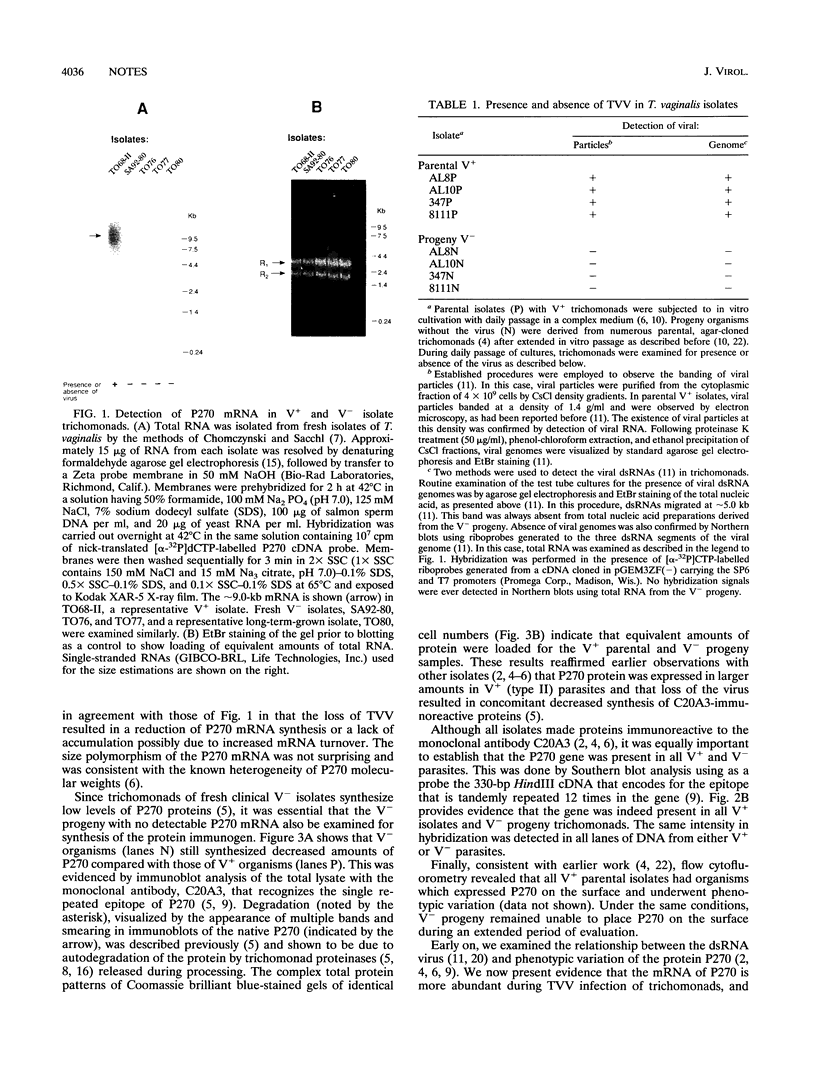
Some isolates of Trichomonas vaginalis carry a double-stranded RNA virus (TVV) and undergo phenotypic variation between surface expression and cytoplasmic expression of a prominent immunogen (P270). Only trichomonads with TVV express P270 on the surface. Northern (RNA) blots using a specific cDNA encoding the repeat element of the phenotypically varying P270 immunogen as a probe showed accumulation of P270 transcript only in isolates with TVV (V+) in contrast to isolates without the virus (V-). To test further the influence of virus infection on P270 mRNA expression, V- progeny, derived from the parental V+ isolates, were tested. Trichomonads of V- progeny, like the fresh clinical V- isolates, also showed no accumulation of P270 mRNA. By immunoblotting with a monoclonal antibody to the key repeated epitope of P270, it was found that V- organisms had quantitatively less immunoreactive protein than the parental V+ isolates. Although V+ and V- isolates contained proteins immunoreactive with the monoclonal antibody to P270, it was necessary to test for the presence of the P270 gene among all the isolates. As expected, Southern blots demonstrated that V+ and V- trichomonads possessed the gene encoding P270. These data suggest that the double-stranded RNA virus of T. vaginalis is involved in the regulation of P270 mRNA accumulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Demes P., Gombosová A., Valent M., Yánoska A., Fabusová H., Kasmala L., Garza G. E., Metcalfe E. C. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun. 1987 May;55(5):1037–1041. doi: 10.1128/iai.55.5.1037-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L., Metcalfe E., Garza G. E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun. 1986 Aug;53(2):285–293. doi: 10.1128/iai.53.2.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect Immun. 1986 Sep;53(3):697–699. doi: 10.1128/iai.53.3.697-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Neale K. A. Relatedness of structures of a major immunogen in Trichomonas vaginalis isolates. Infect Immun. 1989 Jun;57(6):1849–1853. doi: 10.1128/iai.57.6.1849-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Trichomonas vaginalis NYH286 phenotypic variation may be coordinated for a repertoire of trichomonad surface immunogens. Infect Immun. 1987 Sep;55(9):1957–1962. doi: 10.1128/iai.55.9.1957-1962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coombs G. H., North M. J. An analysis of the proteinases of Trichomonas vaginalis by polyacrylamide gel electrophoresis. Parasitology. 1983 Feb;86(Pt 1):1–6. doi: 10.1017/s0031182000057103. [DOI] [PubMed] [Google Scholar]
- Dailey D. C., Alderete J. F. The phenotypically variable surface protein of Trichomonas vaginalis has a single, tandemly repeated immunodominant epitope. Infect Immun. 1991 Jun;59(6):2083–2088. doi: 10.1128/iai.59.6.2083-2088.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- Khoshnan A., Alderete J. F. Multiple double-stranded RNA segments are associated with virus particles infecting Trichomonas vaginalis. J Virol. 1993 Dec;67(12):6950–6955. doi: 10.1128/jvi.67.12.6950-6955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. G., Choi G. H., Nuss D. L. Regulatory pathways governing modulation of fungal gene expression by a virulence-attenuating mycovirus. EMBO J. 1992 Dec;11(12):4539–4548. doi: 10.1002/j.1460-2075.1992.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale K. A., Alderete J. F. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect Immun. 1990 Jan;58(1):157–162. doi: 10.1128/iai.58.1.157-162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
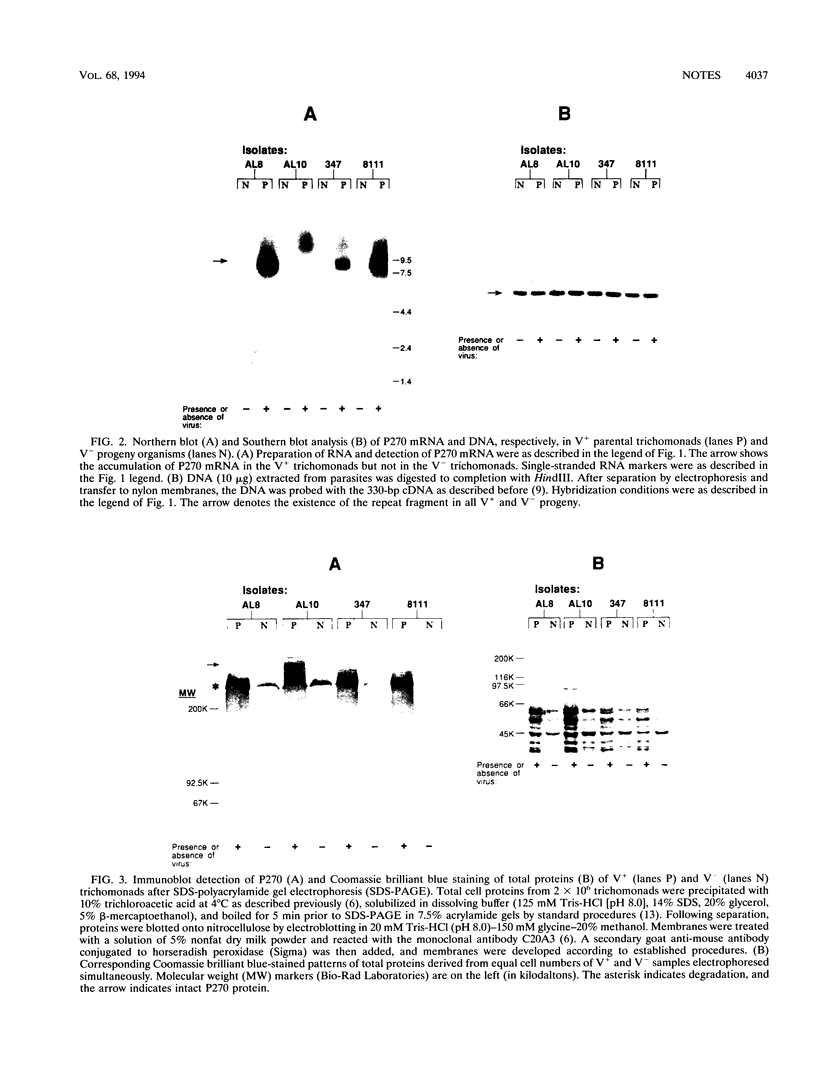
- Nuss D. L. Biological control of chestnut blight: an example of virus-mediated attenuation of fungal pathogenesis. Microbiol Rev. 1992 Dec;56(4):561–576. doi: 10.1128/mr.56.4.561-576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K. D., Weeks R., Guilbride L., Myler P. J. Molecular organization of Leishmania RNA virus 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8596–8600. doi: 10.1073/pnas.89.18.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7956–7960. doi: 10.1073/pnas.83.20.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Viruses of the protozoa. Annu Rev Microbiol. 1991;45:251–263. doi: 10.1146/annurev.mi.45.100191.001343. [DOI] [PubMed] [Google Scholar]
- Wang A., Wang C. C., Alderete J. F. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J Exp Med. 1987 Jul 1;166(1):142–150. doi: 10.1084/jem.166.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]