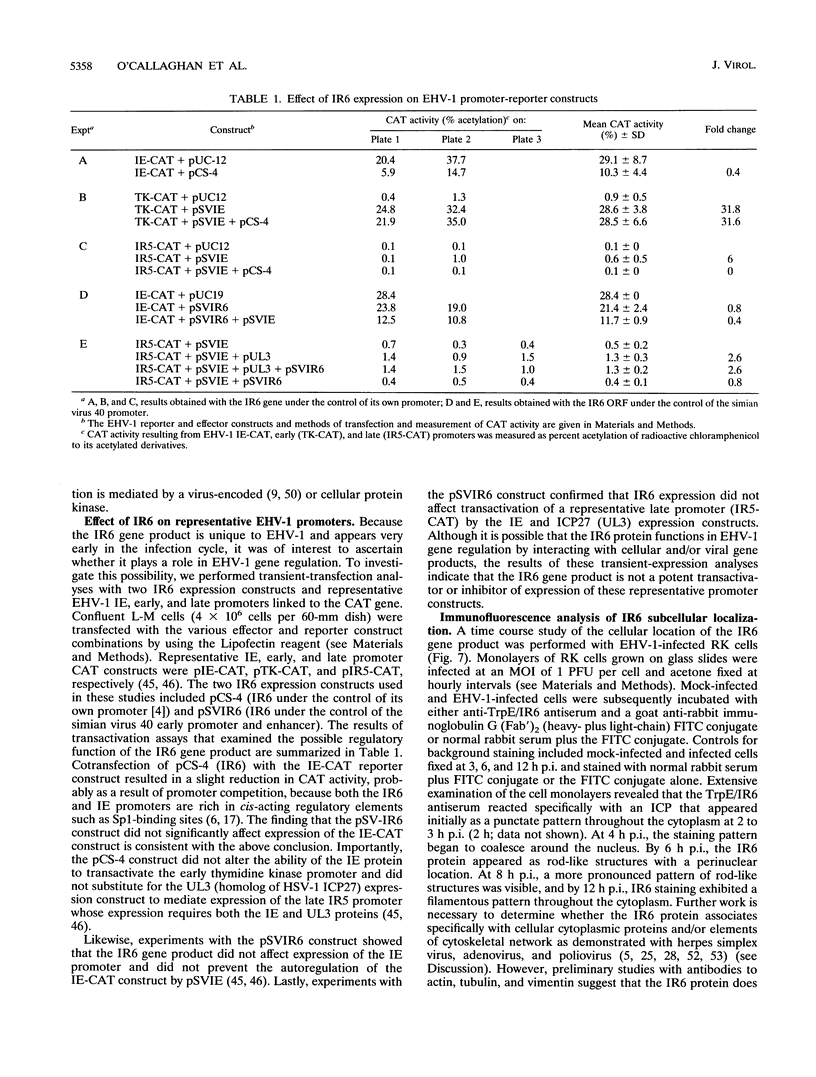
Abstract
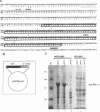
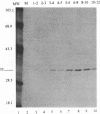
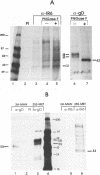
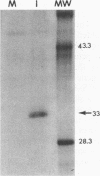
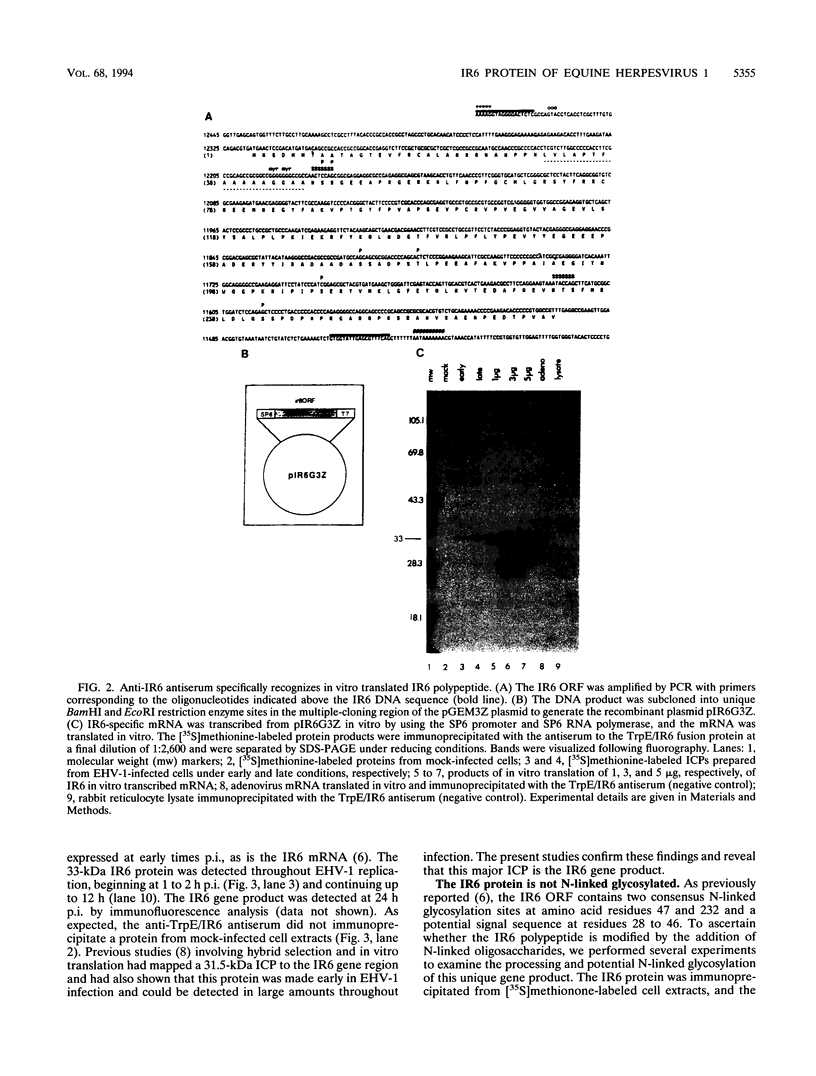
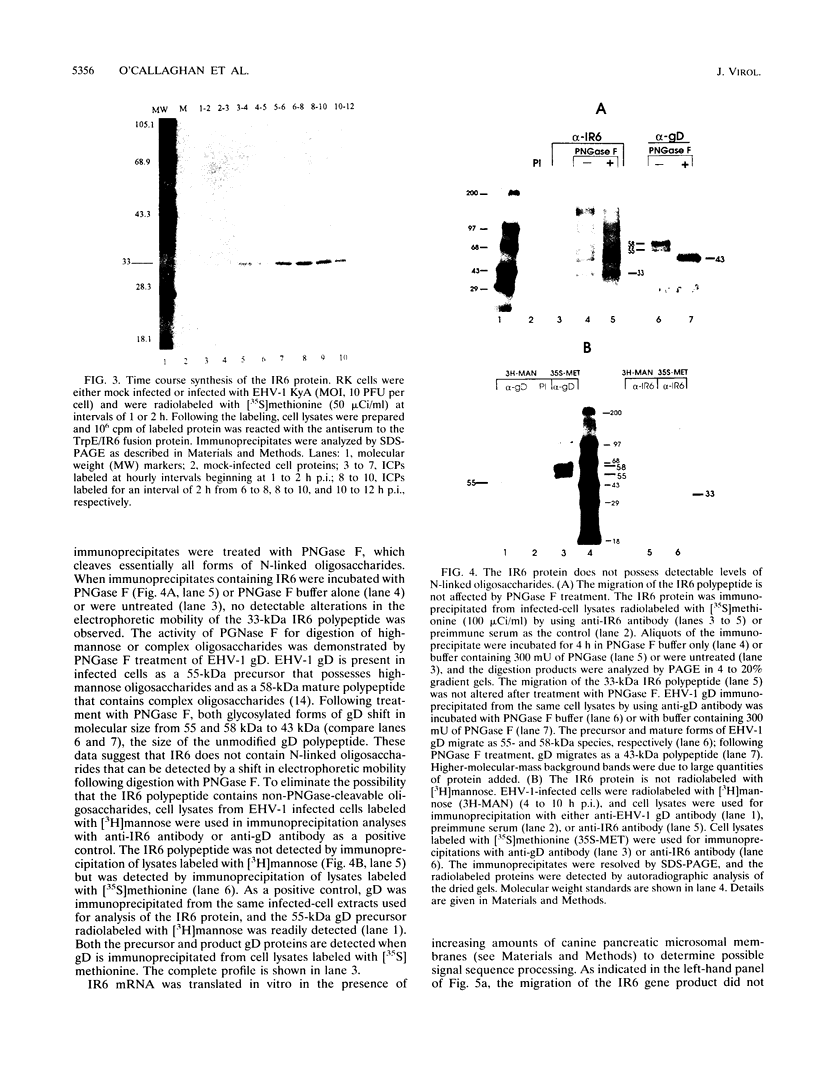
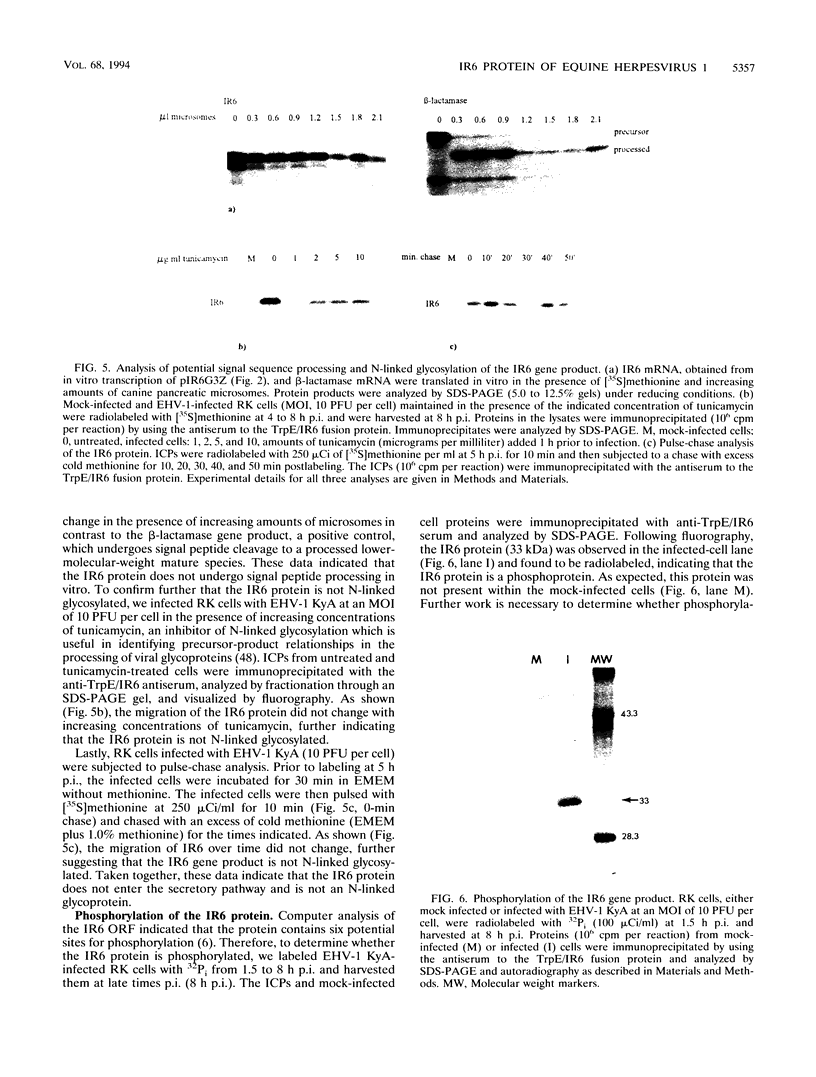
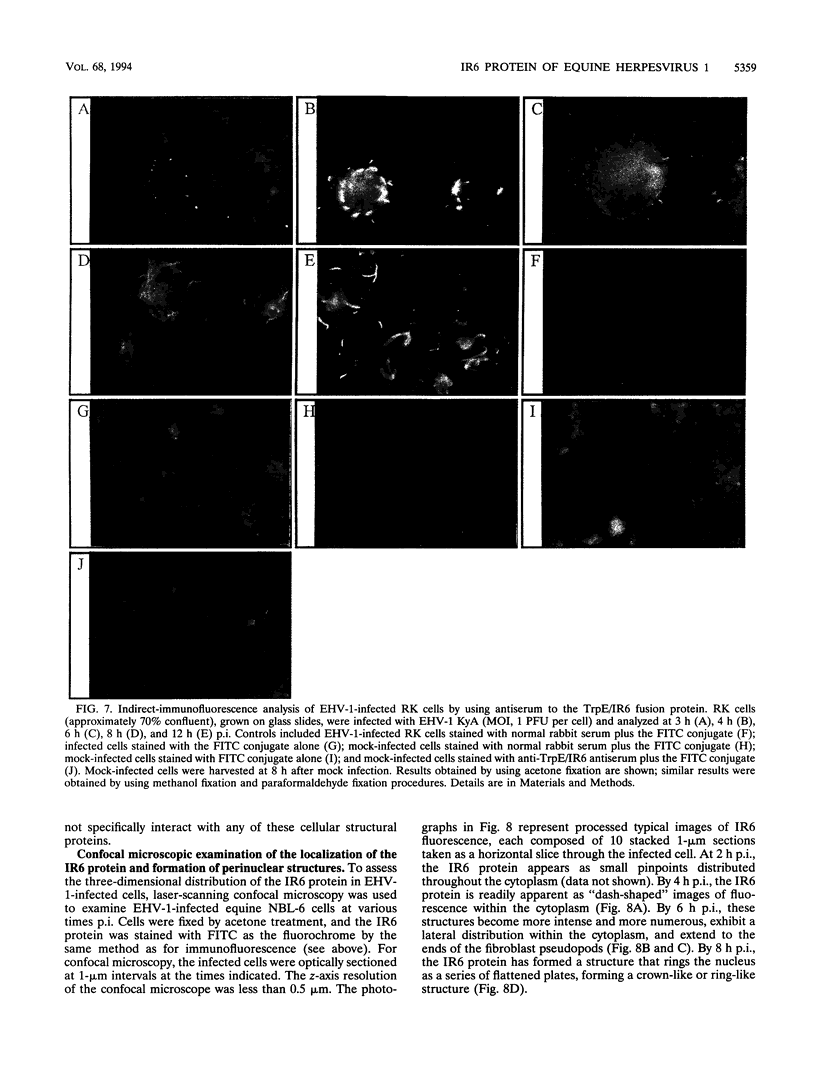
The IR6 gene of equine herpesvirus 1 (EHV-1) is a novel gene that maps within each inverted repeat (IR), encodes a potential protein of 272 amino acids, and is expressed as a 1.2-kb RNA whose synthesis begins at very early times (1.5 h) after infection and continues throughout the infection cycle (C. A. Breeden, R. R. Yalamanchili, C.F. Colle, and D.J. O'Callaghan, Virology 191:649-660,1992). To identify the IR6 protein and ascertain its properties, we generated an IR6-specific polyclonal antiserum to a TrpE/IR6 fusion protein containing 129 amino acids (residues 134 to 262) of the IR6 protein. This antiserum immunoprecipitated a 33-kDa protein generated by in vitro translation of mRNA transcribed from a pGEM construct (IR6/pGEM-3Z) that contains the entire IR6 open reading frame. The anti-IR6 antibody also recognized an infected-cell protein of approximately 33 kDa that was expressed as early as 1 to 2 h postinfection and was synthesized throughout the infection cycle. A variety of biochemical analyses including radiolabeling the IR6 protein with oligosaccharide precursors, translation of IR6 mRNA in the presence of canine pancreatic microsomes, radiolabeling the IR6 protein in the presence of tunicamycin, and pulse-chase labeling experiments indicated that the two potential sites for N-linked glycosylation were not used and that the IR6 protein does not enter the secretory pathway. To address the possibility that the unique IR6 gene encodes a novel regulatory protein, we transiently transfected an IR6 expression construct into L-M fibroblasts alone or with an immediate-early gene expression construct along with a representative EHV-1 immediate-early, early, or late promoter-chloramphenicol acetyltransferase reporter construct. The results indicated that the IR6 protein does not affect the expression of these representative promoter constructs. Interestingly, the IR6 protein was shown to be phosphorylated and to associate with purified EHV-1 virions and nucleocapsids. Lastly, immunofluorescence and laser-scanning confocal microscopic analyses revealed that the IR6 protein is distributed throughout the cytoplasm at early times postinfection and that by 4 to 6 h it appears as "dash-shaped" structures that localize to the perinuclear region. At late times after infection (8 to 12 h), these structures assemble around the nucleus, and three-dimensional image analyses reveal that the IR6 protein forms a crown-like structure that surrounds the nucleus as a perinuclear network.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius C. T., Nagesha H. S., Studdert M. J. Equine herpesvirus 5: comparisons with EHV2 (equine cytomegalovirus), cloning, and mapping of a new equine herpesvirus with a novel genome structure. Virology. 1992 Nov;191(1):176–186. doi: 10.1016/0042-6822(92)90179-s. [DOI] [PubMed] [Google Scholar]
- Audonnet J. C., Winslow J., Allen G., Paoletti E. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI and gE. J Gen Virol. 1990 Dec;71(Pt 12):2969–2978. doi: 10.1099/0022-1317-71-12-2969. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Three-dimensional structures of maturable and abortive capsids of equine herpesvirus 1 from cryoelectron microscopy. J Virol. 1990 Feb;64(2):563–573. doi: 10.1128/jvi.64.2.563-573.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann R. P., Staczek J., O'Callaghan D. J. Equine herpesvirus type 1 defective-interfering (DI) particle DNA structure: the central region of the inverted repeat is deleted from DI DNA. Virology. 1987 Jul;159(1):137–146. doi: 10.1016/0042-6822(87)90356-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Abulafia R., Bratosin S. Herpes simplex virus and protein transport are associated with the cytoskeletal framework and the nuclear matrix in infected BSC-1 cells. Virology. 1983 Sep;129(2):501–507. doi: 10.1016/0042-6822(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Breeden C. A., Yalamanchili R. R., Colle C. F., O'Callaghan D. J. Identification and transcriptional mapping of genes encoded at the IR/Us junction of equine herpesvirus type 1. Virology. 1992 Dec;191(2):649–660. doi: 10.1016/0042-6822(92)90240-p. [DOI] [PubMed] [Google Scholar]
- Caughman G. B., Staczek J., O'Callaghan D. J. Equine herpesvirus type 1 infected cell polypeptides: evidence for immediate early/early/late regulation of viral gene expression. Virology. 1985 Aug;145(1):49–61. doi: 10.1016/0042-6822(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Colle C. F., 3rd, Flowers C. C., O'Callaghan D. J. Open reading frames encoding a protein kinase, homolog of glycoprotein gX of pseudorabies virus, and a novel glycoprotein map within the unique short segment of equine herpesvirus type 1. Virology. 1992 Jun;188(2):545–557. doi: 10.1016/0042-6822(92)90509-n. [DOI] [PubMed] [Google Scholar]
- Elton D. M., Halliburton I. W., Killington R. A., Meredith D. M., Bonass W. A. Sequence analysis of the 4.7-kb BamHI-EcoRI fragment of the equine herpesvirus type-1 short unique region. Gene. 1991 May 30;101(2):203–208. doi: 10.1016/0378-1119(91)90412-5. [DOI] [PubMed] [Google Scholar]
- Flowers C. C., Eastman E. M., O'Callaghan D. J. Sequence analysis of a glycoprotein D gene homolog within the unique short segment of the EHV-1 genome. Virology. 1991 Jan;180(1):175–184. doi: 10.1016/0042-6822(91)90021-3. [DOI] [PubMed] [Google Scholar]
- Flowers C. C., O'Callaghan D. J. Equine herpesvirus 1 glycoprotein D: mapping of the transcript and a neutralization epitope. J Virol. 1992 Nov;66(11):6451–6460. doi: 10.1128/jvi.66.11.6451-6460.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers C. C., O'Callaghan D. J. The equine herpesvirus type 1 (EHV-1) homolog of herpes simplex virus type 1 US9 and the nature of a major deletion within the unique short segment of the EHV-1 KyA strain genome. Virology. 1992 Sep;190(1):307–315. doi: 10.1016/0042-6822(92)91217-i. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., Caughman G. B., O'Callaghan D. J., Staczek J. Regulation of equine herpesvirus type 1 gene expression: characterization of immediate early, early, and late transcription. Virology. 1987 May;158(1):79–87. doi: 10.1016/0042-6822(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., O'Callaghan D. J., Staczek J. Characterization and mapping of equine herpesvirus type 1 immediate early, early, and late transcripts. Virus Res. 1987 Sep;8(3):233–244. doi: 10.1016/0168-1702(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Harty R. N., Caughman G. B., Holden V. R., O'Callaghan D. J. Characterization of the myristylated polypeptide encoded by the UL1 gene that is conserved in the genome of defective interfering particles of equine herpesvirus 1. J Virol. 1993 Jul;67(7):4122–4132. doi: 10.1128/jvi.67.7.4122-4132.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty R. N., O'Callaghan D. J. An early gene maps within and is 3' coterminal with the immediate-early gene of equine herpesvirus 1. J Virol. 1991 Jul;65(7):3829–3838. doi: 10.1128/jvi.65.7.3829-3838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty R. N., O'Callaghan D. J. Identification and expression of the UL1 gene product of equine herpesvirus 1. Virus Res. 1992 Sep 1;25(1-2):105–116. doi: 10.1016/0168-1702(92)90103-g. [DOI] [PubMed] [Google Scholar]
- Holden V. R., Harty R. N., Yalamanchili R. R., O'Callaghan D. J. The IR3 gene of equine herpesvirus type 1: a unique gene regulated by sequences within the intron of the immediate-early gene. DNA Seq. 1992;3(3):143–152. doi: 10.3109/10425179209034010. [DOI] [PubMed] [Google Scholar]
- Holden V. R., Yalamanchili R. R., Harty R. N., O'Callaghan D. J. ICP22 homolog of equine herpesvirus 1: expression from early and late promoters. J Virol. 1992 Feb;66(2):664–673. doi: 10.1128/jvi.66.2.664-673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden V. R., Yalamanchili R. R., Harty R. N., O'Callaghan D. J. Identification and characterization of an equine herpesvirus 1 late gene encoding a potential zinc finger. Virology. 1992 Jun;188(2):704–713. doi: 10.1016/0042-6822(92)90525-t. [DOI] [PubMed] [Google Scholar]
- Joachims M., Etchison D. Poliovirus infection results in structural alteration of a microtubule-associated protein. J Virol. 1992 Oct;66(10):5797–5804. doi: 10.1128/jvi.66.10.5797-5804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Leung-Tack P., Audonnet J. C., Riviere M. The complete DNA sequence and the genetic organization of the short unique region (US) of the bovine herpesvirus type 1 (ST strain). Virology. 1994 Mar;199(2):409–421. doi: 10.1006/viro.1994.1139. [DOI] [PubMed] [Google Scholar]
- McNabb D. S., Courtney R. J. Identification and characterization of the herpes simplex virus type 1 virion protein encoded by the UL35 open reading frame. J Virol. 1992 May;66(5):2653–2663. doi: 10.1128/jvi.66.5.2653-2663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesha H. S., Crabb B. S., Studdert M. J. Analysis of the nucleotide sequence of five genes at the left end of the unique short region of the equine herpesvirus 4 genome. Arch Virol. 1993;128(1-2):143–154. doi: 10.1007/BF01309795. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C., Booy F. P., Steven A. C. Nucleocapsid mass and capsomer protein stoichiometry in equine herpesvirus 1: scanning transmission electron microscopic study. J Virol. 1989 Sep;63(9):3777–3783. doi: 10.1128/jvi.63.9.3777-3783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991 Feb;65(2):613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Cheevers W. P., Gentry G. A., Randall C. C. Kinetics of cellular and viral DNA synthesis in equine abortion (herpes) virus infection of L-M cells. Virology. 1968 Sep;36(1):104–114. doi: 10.1016/0042-6822(68)90120-7. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. T., Caughman G. B., Gray W. L., Baumann R. P., Staczek J., O'Callaghan D. J. Analysis of the in vitro translation products of the equine herpesvirus type 1 immediate early mRNA. Virology. 1988 Oct;166(2):451–462. doi: 10.1016/0042-6822(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Chen L. B., Fields B. N. The interaction of mammalian reoviruses with the cytoskeleton of monkey kidney CV-1 cells. Virology. 1982 Jul 30;120(2):399–411. doi: 10.1016/0042-6822(82)90040-x. [DOI] [PubMed] [Google Scholar]
- Smith R. H., Caughman G. B., O'Callaghan D. J. Characterization of the regulatory functions of the equine herpesvirus 1 immediate-early gene product. J Virol. 1992 Feb;66(2):936–945. doi: 10.1128/jvi.66.2.936-945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. H., Zhao Y., O'Callaghan D. J. The equine herpesvirus 1 (EHV-1) UL3 gene, an ICP27 homolog, is necessary for full activation of gene expression directed by an EHV-1 late promoter. J Virol. 1993 Feb;67(2):1105–1109. doi: 10.1128/jvi.67.2.1105-1109.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sun Y., Brown S. M. The open reading frames 1, 2, 71, and 75 are nonessential for the replication of equine herpesvirus type 1 in vitro. Virology. 1994 Mar;199(2):448–452. doi: 10.1006/viro.1994.1143. [DOI] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- White E., Cipriani R. Role of adenovirus E1B proteins in transformation: altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein. Mol Cell Biol. 1990 Jan;10(1):120–130. doi: 10.1128/mcb.10.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R. Specific disruption of intermediate filaments and the nuclear lamina by the 19-kDa product of the adenovirus E1B oncogene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9886–9890. doi: 10.1073/pnas.86.24.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F., Courtney R. J. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J Virol. 1989 Aug;63(8):3338–3344. doi: 10.1128/jvi.63.8.3338-3344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Holden V. R., Harty R. N., O'Callaghan D. J. Identification and transcriptional analyses of the UL3 and UL4 genes of equine herpesvirus 1, homologs of the ICP27 and glycoprotein K genes of herpes simplex virus. J Virol. 1992 Sep;66(9):5363–5372. doi: 10.1128/jvi.66.9.5363-5372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]