Abstract
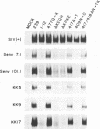
The determinants of immune recognition by five monoclonal antibodies (KK5, KK9, KK17, Senv7.1, and Senv101.1) that neutralize simian immunodeficiency virus infectivity were analyzed. These five neutralizing monoclonal antibodies were generated to native SIVmac251 envelope glycoprotein expressed by a vaccinia virus recombinant vector. All five recognize conformational or discontinuous epitopes and require native antigen for optimal recognition. These monoclonal antibodies also recognize SIVmac239 gp120, but they do not recognize gp120 of two natural variants of SIVmac239, 1-12 and 8-22, which evolved during the course of persistent infection in vivo (D.P.W. Burns and R.C. Desrosiers, J. Virol. 65:1843-1854, 1991). Recombinant viruses which were constructed by exchanging variable regions between SIVmac239 and variant 1-12 were used to define domains important for recognition. Radioimmunoprecipitation analysis demonstrated that sequence changes in variable regions 4 and 5 (V4/V5) were primarily responsible for the loss of recognition of the 1-12 variant. Site-specific mutants were used to define precise changes that eliminate recognition by these neutralizing antibodies. Changing N-409 to D, deletion of KPKE, and deletion of KEQH in V4 each resulted in loss of recognition by all five monoclonal antibodies. SIVs with these natural sequence changes are still replication competent and viable. Changing A-417 to T or A/N-417/418 to TK in V4 or Q-477 to K in V5 did not alter recognition detectably. These results define specific, naturally occurring sequence changes in V4 of SIVmac that result in loss of recognition by one class of SIVmac neutralizing antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Abrahamsson B., Nagy K., Aurelius E., Gaines H., Nyström G., Fenyö E. M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990 Feb;4(2):107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Benichou S., Legrand R., Nakagawa N., Faure T., Traincard F., Vogt G., Dormont D., Tiollais P., Kieny M. P., Madaule P. Identification of a neutralizing domain in the external envelope glycoprotein of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1992 Jun;8(6):1165–1170. doi: 10.1089/aid.1992.8.1165. [DOI] [PubMed] [Google Scholar]
- Berkower I., Murphy D., Smith C. C., Smith G. E. A predominant group-specific neutralizing epitope of human immunodeficiency virus type 1 maps to residues 342 to 511 of the envelope glycoprotein gp120. J Virol. 1991 Nov;65(11):5983–5990. doi: 10.1128/jvi.65.11.5983-5990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. P., Collignon C., Desrosiers R. C. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J Virol. 1993 Jul;67(7):4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Knowles D. P., Jr, Norton L. K. Neutralization-resistant antigenic variants of caprine arthritis-encephalitis lentivirus associated with progressive arthritis. J Infect Dis. 1991 Oct;164(4):679–685. doi: 10.1093/infdis/164.4.679. [DOI] [PubMed] [Google Scholar]
- D'Souza M. P., Kent K. A., Thiriart C., Collignon C., Milman G. International collaboration comparing neutralization and binding assays for monoclonal antibodies to simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1993 May;9(5):415–422. doi: 10.1089/aid.1993.9.415. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Ellis T. M., Wilcox G. E., Robinson W. F. Antigenic variation of caprine arthritis-encephalitis virus during persistent infection of goats. J Gen Virol. 1987 Dec;68(Pt 12):3145–3152. doi: 10.1099/0022-1317-68-12-3145. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., Montefiori D. C., Kent K. A., Ryan K. A., Wyman P. D., Stott J., Bolognesi D. P., Murphey-Corb M., Larosa G. J. Studies of the conformation-dependent neutralizing epitopes of simian immunodeficiency virus envelope protein. J Virol. 1994 Apr;68(4):2624–2631. doi: 10.1128/jvi.68.4.2624-2631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., Schmidt S., Kaufmann M., Cates N., Langedijk J. P., Meloen R. H., Desrosiers R. C., Burns D. P., Bolognesi D. P. The principal neutralization determinant of simian immunodeficiency virus differs from that of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1418–1422. doi: 10.1073/pnas.89.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Hamm T. E., Goldstein S., Kitov S., Hirsch V. M. The genetic fate of molecularly cloned simian immunodeficiency virus in experimentally infected macaques. Virology. 1991 Nov;185(1):217–228. doi: 10.1016/0042-6822(91)90769-8. [DOI] [PubMed] [Google Scholar]
- Kent K. A., Gritz L., Stallard G., Cranage M. P., Collignon C., Thiriart C., Corcoran T., Silvera P., Stott E. J. Production and of monoclonal antibodies to simian immunodeficiency virus envelope glycoproteins. AIDS. 1991 Jul;5(7):829–836. doi: 10.1097/00002030-199107000-00006. [DOI] [PubMed] [Google Scholar]
- Kent K. A., Rud E., Corcoran T., Powell C., Thiriart C., Collignon C., Stott E. J. Identification of two neutralizing and 8 non-neutralizing epitopes on simian immunodeficiency virus envelope using monoclonal antibodies. AIDS Res Hum Retroviruses. 1992 Jun;8(6):1147–1151. doi: 10.1089/aid.1992.8.1147. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Kodama T., Burns D. P., Silva D. P., Veronese F. D., Desrosiers R. C. Strain-specific neutralizing determinant in the transmembrane protein of simian immunodeficiency virus. J Virol. 1991 Apr;65(4):2010–2018. doi: 10.1128/jvi.65.4.2010-2018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y. Antigenic variation of equine infectious anemia virus as detected by virus neutralization. Brief report. Arch Virol. 1988;98(1-2):91–97. doi: 10.1007/BF01321009. [DOI] [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41(1):1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- Lutley R., Pétursson G., Pálsson P. A., Georgsson G., Klein J., Nathanson N. Antigenic drift in visna: virus variation during long-term infection of Icelandic sheep. J Gen Virol. 1983 Jul;64(Pt 7):1433–1440. doi: 10.1099/0022-1317-64-7-1433. [DOI] [PubMed] [Google Scholar]
- McBride B. W., Corthals G., Rud E., Kent K., Webster S., Cook N., Cranage M. P. Comparison of serum antibody reactivities to a conformational and to linear antigenic sites in the external envelope glycoprotein of simian immunodeficiency virus (SIVmac) induced by infection and vaccination. J Gen Virol. 1993 Jun;74(Pt 6):1033–1041. doi: 10.1099/0022-1317-74-6-1033. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Norton L. K., O'Rourke K. I., Cheevers W. P. Antigenic variation of neutralization-sensitive epitopes of caprine arthritis-encephalitis lentivirus during persistent arthritis. J Virol. 1988 Sep;62(9):3488–3492. doi: 10.1128/jvi.62.9.3488-3492.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Moore J. P., Ho D. D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993 Feb;67(2):863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Nara P. L. The role of the V3 loop of gp120 in HIV infection. AIDS. 1991;5 (Suppl 2):S21–S33. doi: 10.1097/00002030-199101001-00004. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Sattentau Q. J., Wyatt R., Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994 Jan;68(1):469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Clements J. E. Virus mutation during 'slow infection': temporal development and characterization of mutants of visna virus recovered from sheep. J Gen Virol. 1978 Nov;41(2):343–352. doi: 10.1099/0022-1317-41-2-343. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M., Papenhausen M. D., Benveniste R. E., Morton W. R. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991 Dec;65(12):7025–7031. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier D. A., Desrosiers R. C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Klasse P. J., McKeating J. A. HIV-1 neutralization directed to epitopes other than linear V3 determinants. AIDS. 1991;5 (Suppl 2):S135–S143. doi: 10.1097/00002030-199101001-00019. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Scandella C. J., Skiles P. V., Haigwood N. L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science. 1991 Oct 4;254(5028):105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- Torres J. V., Malley A., Banapour B., Anderson D. E., Axthelm M. K., Gardner M. B., Benjamini E. An epitope on the surface envelope glycoprotein (gp130) of simian immunodeficiency virus (SIVmac) involved in viral neutralization and T cell activation. AIDS Res Hum Retroviruses. 1993 May;9(5):423–430. doi: 10.1089/aid.1993.9.423. [DOI] [PubMed] [Google Scholar]
- Tremblay M., Wainberg M. A. Neutralization of multiple HIV-1 isolates from a single subject by autologous sequential sera. J Infect Dis. 1990 Sep;162(3):735–737. doi: 10.1093/infdis/162.3.735. [DOI] [PubMed] [Google Scholar]
- di Marzo Veronese F., Reitz M. S., Jr, Gupta G., Robert-Guroff M., Boyer-Thompson C., Louie A., Gallo R. C., Lusso P. Loss of a neutralizing epitope by a spontaneous point mutation in the V3 loop of HIV-1 isolated from an infected laboratory worker. J Biol Chem. 1993 Dec 5;268(34):25894–25901. [PubMed] [Google Scholar]