Abstract
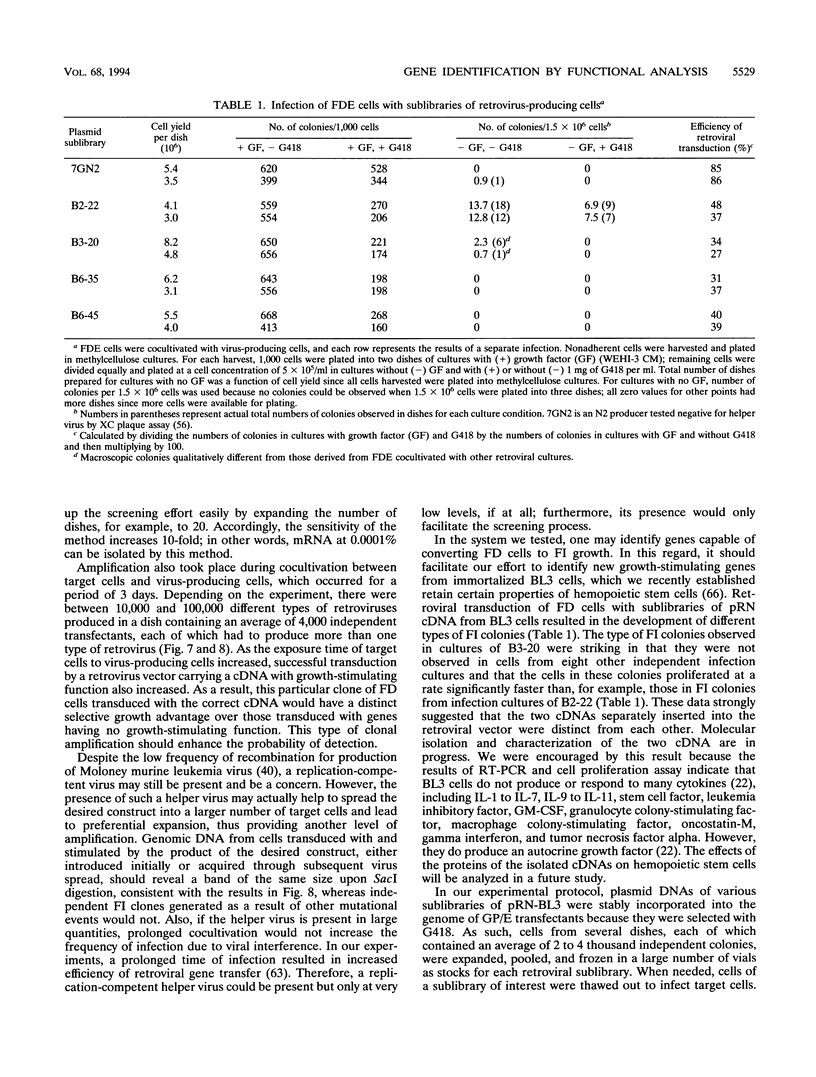
Retroviral gene transfer efficiently delivers genes of interest stably into target cells, and expression cDNA cloning has been shown to be highly successful. Considering these two advantages, we now report a method by which one can identify genes stimulating cell growth through functional analysis. The first step requires the construction of a retroviral cDNA expression library and the optimization of transfection of vector DNA into virus packaging cells. The second step involves the cocultivation of target cells with libraries of retrovirus-producing cells, resulting in the amplification of target cells transduced with a gene(s) stimulating cell growth. Under standardized conditions of transfection, we detected an average of 4,000 independent clones per dish, among which expression of a retroviral beta-galactosidase gene at an abundance of 0.2% could be detected. Next, we demonstrated the augmentation of the sensitivity of the assay by retroviral infection and functional analysis. We did this by cocultivating factor-dependent (FD) cells with dishes of GP/E cells transfected with plasmids containing various molar ratios of pN2-IL3 DNA and retroviral library cDNA and by determining the highest dilution of pN2-IL3 which still resulted in the conversion of FD cells to factor independence. The retroviral interleukin-3 gene at an abundance as low as 0.001% could be detected. Indeed, we were able to detect from FD cells the development of factor-independent colonies with different phenotypes after retroviral transfer of cDNAs from an immortalized hemopoietic stem cell line. Thus, the combination of a standardized high-efficiency DNA transfection and retrovirus-mediated gene transfer should facilitate the identification of genes capable of conferring to target FD cells a detectable new function or phenotype. By scaling up the size of the experiment realistically during screening, the assay can detect cDNA at an abundance of lower than 0.0001%.
Full text
PDF
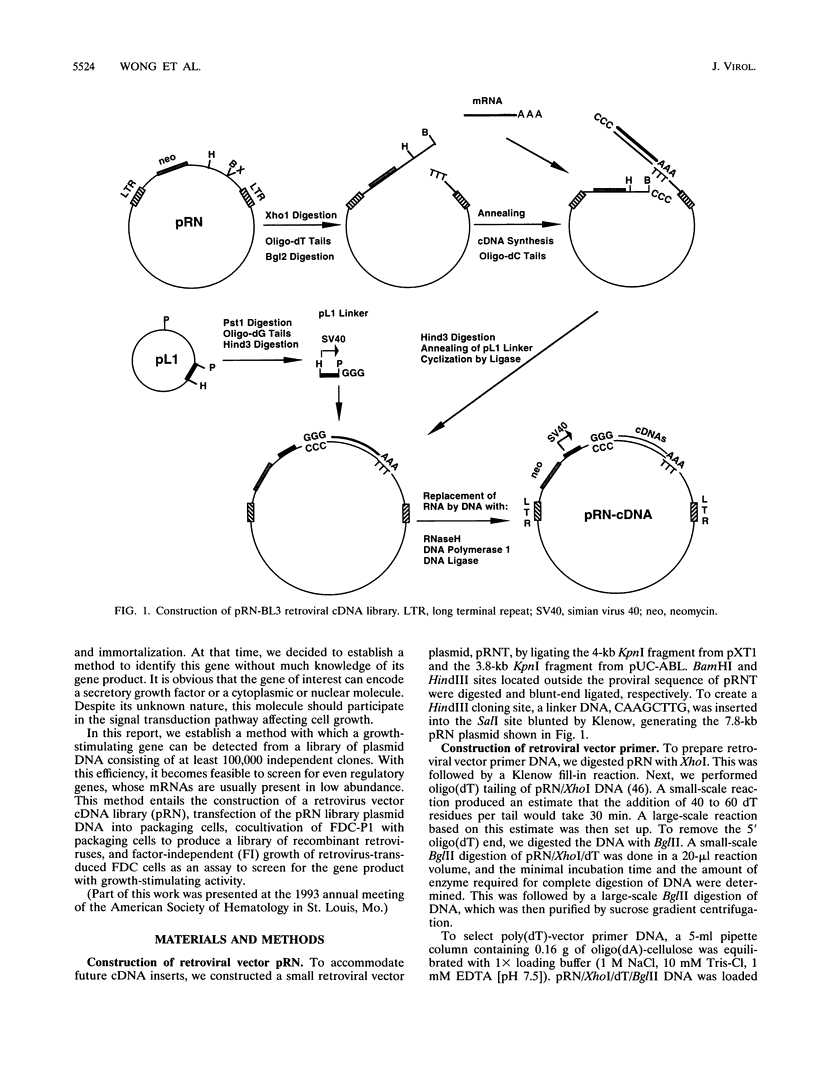

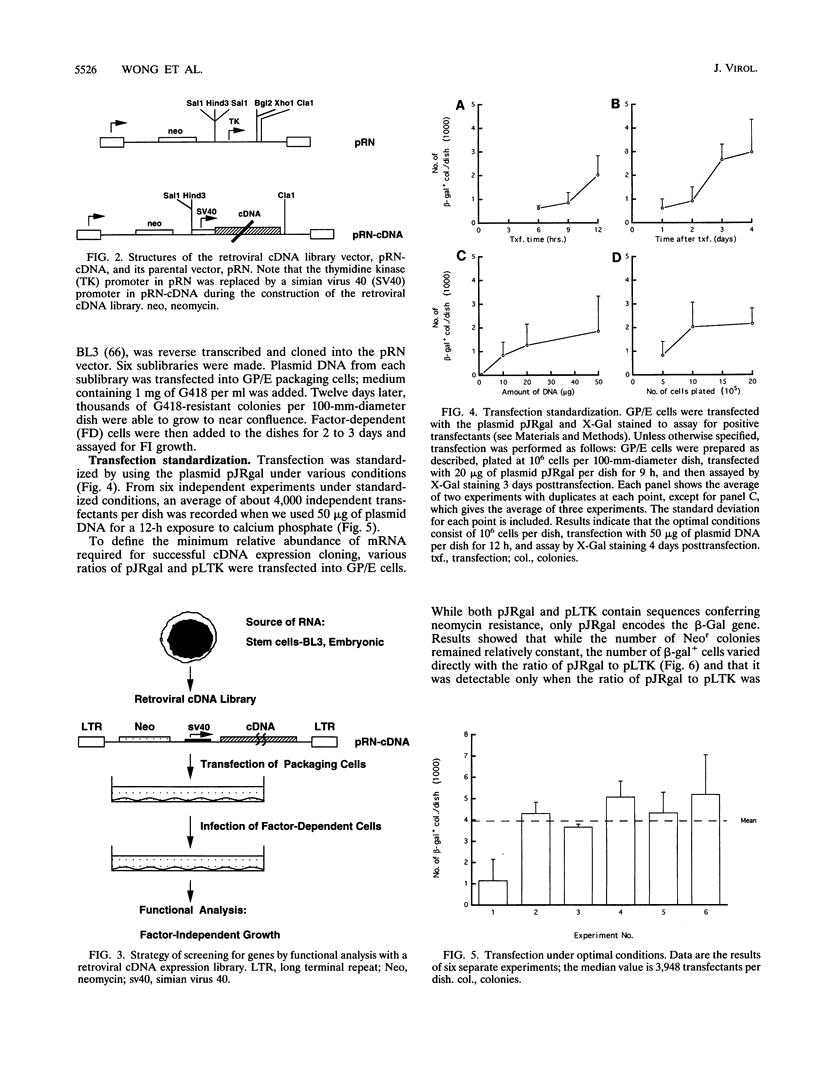
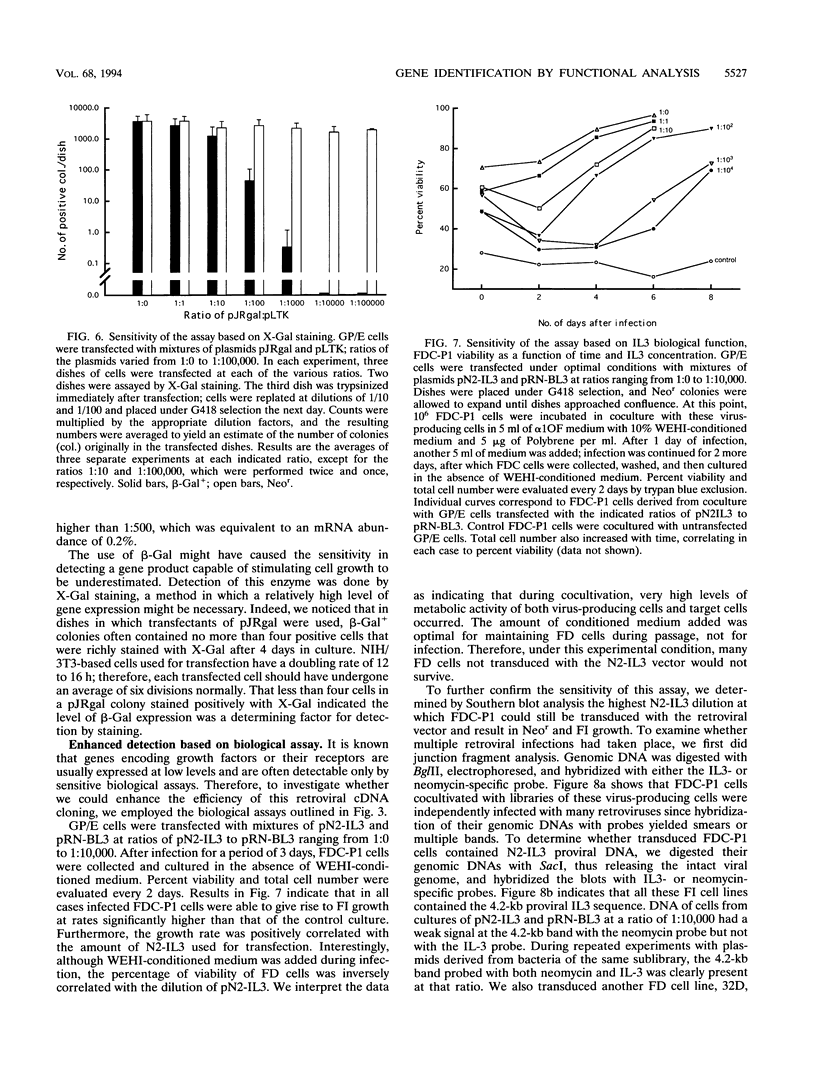
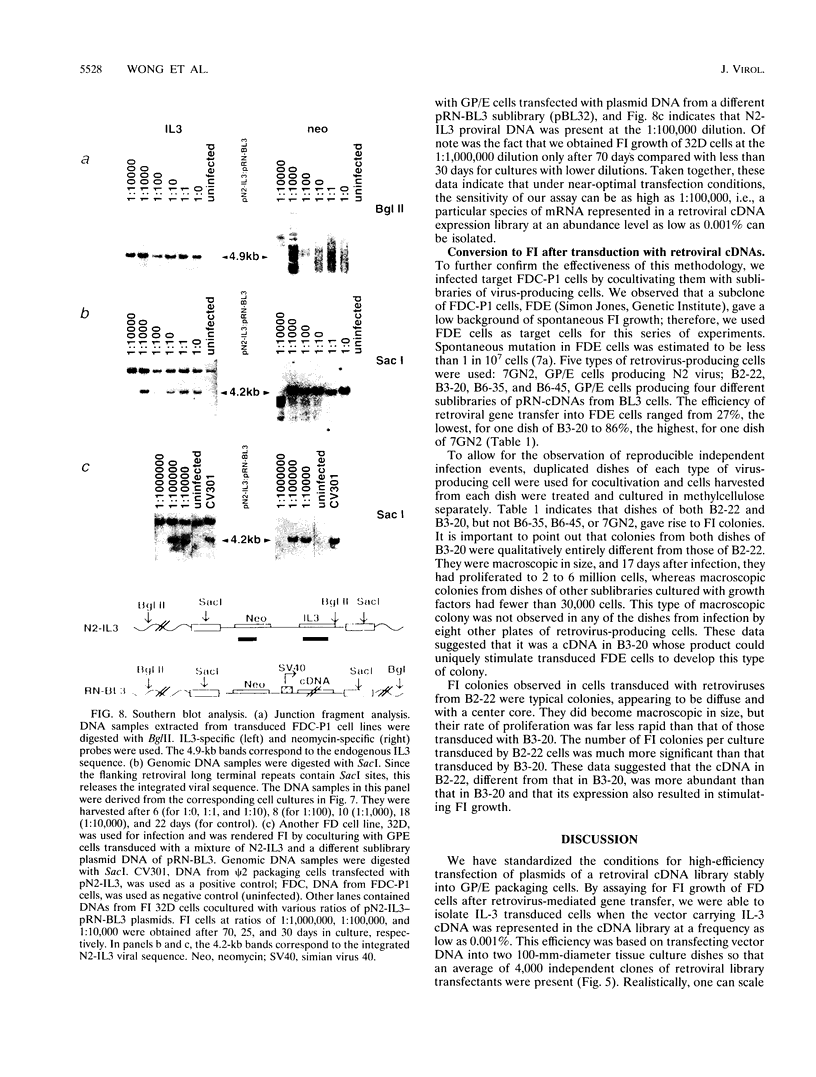


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont J. W., Henkel-Tigges J., Chang S. M., Wager-Smith K., Kellems R. E., Dick J. E., Magli M. C., Phillips R. A., Bernstein A., Caskey C. T. Expression of human adenosine deaminase in murine haematopoietic progenitor cells following retroviral transfer. Nature. 1986 Jul 24;322(6077):385–387. doi: 10.1038/322385a0. [DOI] [PubMed] [Google Scholar]
- Blasi E., Mathieson B. J., Varesio L., Cleveland J. L., Borchert P. A., Rapp U. R. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985 Dec 19;318(6047):667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- Boswell H. S., Nahreini T. S., Burgess G. S., Srivastava A., Gabig T. G., Inhorn L., Srour E. F., Harrington M. A. A RAS oncogene imparts growth factor independence to myeloid cells that abnormally regulate protein kinase C: a nonautocrine transformation pathway. Exp Hematol. 1990 Jun;18(5):452–460. [PubMed] [Google Scholar]
- Boulter C. A., Wagner E. F. The effects of v-src expression on the differentiation of embryonal carcinoma cells. Oncogene. 1988 Mar;2(3):207–214. [PubMed] [Google Scholar]
- Browder T. M., Abrams J. S., Wong P. M., Nienhuis A. W. Mechanism of autocrine stimulation in hematopoietic cells producing interleukin-3 after retrovirus-mediated gene transfer. Mol Cell Biol. 1989 Jan;9(1):204–213. doi: 10.1128/mcb.9.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Lang R. A., Gonda T. J., Johnson G. R. Nonneoplastic hematopoietic myeloproliferative syndrome induced by dysregulated multi-CSF (IL-3) expression. Blood. 1989 May 1;73(6):1487–1497. [PubMed] [Google Scholar]
- Chung S. W., Wong P. M., Durkin H., Wu Y. S., Petersen J. Leukemia initiated by hemopoietic stem cells expressing the v-abl oncogene. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1585–1589. doi: 10.1073/pnas.88.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. W., Wong P. M., Shen-Ong G., Ruscetti S., Ishizaka T., Eaves C. J. Production of granulocyte-macrophage colony-stimulating factor by Abelson virus-induced tumorigenic mast cell lines. Blood. 1986 Nov;68(5):1074–1081. [PubMed] [Google Scholar]
- Cone R. D., Reilly E. B., Eisen H. N., Mulligan R. C. Tissue-specific expression of functionally rearranged lambda 1 Ig gene through a retrovirus vector. Science. 1987 May 22;236(4804):954–957. doi: 10.1126/science.3107128. [DOI] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Dean M., Cleveland J. L., Rapp U. R., Ihle J. N. Role of myc in the abrogation of IL3 dependence of myeloid FDC-P1 cells. Oncogene Res. 1987 Aug;1(3):279–296. [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Friedman R. L. Expression of human adenosine deaminase using a transmissable murine retrovirus vector system. Proc Natl Acad Sci U S A. 1985 Feb;82(3):703–707. doi: 10.1073/pnas.82.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Seto Y., Nagata S. Expression cloning of a receptor for murine granulocyte colony-stimulating factor. Cell. 1990 Apr 20;61(2):341–350. doi: 10.1016/0092-8674(90)90814-u. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Ramsay R. G., Johnson G. R. Murine myeloid cell lines derived by in vitro infection with recombinant c-myb retroviruses express myb from rearranged vector proviruses. EMBO J. 1989 Jun;8(6):1767–1775. doi: 10.1002/j.1460-2075.1989.tb03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber H. E., Finley K. D., Hershberg R. M., Katzman S. S., Laikind P. K., Seegmiller J. E., Friedmann T., Yee J. K., Jolly D. J. Retroviral vector-mediated gene transfer into human hematopoietic progenitor cells. Science. 1985 Nov 29;230(4729):1057–1061. doi: 10.1126/science.3864246. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Heard J. M., Roussel M. F., Rettenmier C. W., Sherr C. J. Multilineage hematopoietic disorders induced by transplantation of bone marrow cells expressing the v-fms oncogene. Cell. 1987 Nov 20;51(4):663–673. doi: 10.1016/0092-8674(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Roussel M. F., Ashmun R. A., Sherr C. J. Transduction of human colony-stimulating factor-1 (CSF-1) receptor into interleukin-3-dependent mouse myeloid cells induces both CSF-1-dependent and factor-independent growth. Mol Cell Biol. 1989 Sep;9(9):4069–4073. doi: 10.1128/mcb.9.9.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Keller J. R., Ruscetti S. K., Ruscetti F. W. Introduction of v-abl oncogene induces monocytic differentiation of an IL-3-dependent myeloid progenitor cell line. Oncogene. 1990 Apr;5(4):549–555. [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Hartley J. W., Klinken S. P., Ruscetti S. K., Morse H. C., 3rd v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros G. S., Gillis S., Watson J. D. Induction of IL 2 responsiveness in a murine IL 3-dependent cell line. J Immunol. 1985 Dec;135(6):4009–4014. [PubMed] [Google Scholar]
- Ledley F. D., Darlington G. J., Hahn T., Woo S. L. Retroviral gene transfer into primary hepatocytes: implications for genetic therapy of liver-specific functions. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5335–5339. doi: 10.1073/pnas.84.15.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., McGinnis-Shelnutt M., Woo S. L. Retroviral-mediated gene transfer of human phenylalanine hydroxylase into NIH 3T3 and hepatoma cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):409–413. doi: 10.1073/pnas.83.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Gemmell L., Larson N., Luh J., Arai K., Rennick D. Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4360–4364. doi: 10.1073/pnas.82.13.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Bernard O. FDC-P1 myeloid cells engineered to express fibroblast growth factor receptor 1 proliferate and differentiate in the presence of fibroblast growth factor and heparin. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3315–3319. doi: 10.1073/pnas.89.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J. A., Steelman L. S., Mayo M. W., Algate P. A., Dellow R. A., Kaleko M. Growth-promoting effects of insulin-like growth factor-1 (IGF-1) on hematopoietic cells: overexpression of introduced IGF-1 receptor abrogates interleukin-3 dependency of murine factor-dependent cells by a ligand-dependent mechanism. Blood. 1991 Aug 15;78(4):921–929. [PubMed] [Google Scholar]
- Meckling-Gill K. A., Yee S. P., Schrader J. W., Pawson T. A retrovirus encoding the v-fps protein-tyrosine kinase induces factor-independent growth and tumorigenicity in FDC-P1 cells. Biochim Biophys Acta. 1992 Oct 6;1137(1):65–72. doi: 10.1016/0167-4889(92)90101-g. [DOI] [PubMed] [Google Scholar]
- Migliaccio A. R., Migliaccio G., D'Andrea A., Baiocchi M., Crotta S., Nicolis S., Ottolenghi S., Adamson J. W. Response to erythropoietin in erythroid subclones of the factor-dependent cell line 32D is determined by translocation of the erythropoietin receptor to the cell surface. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11086–11090. doi: 10.1073/pnas.88.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Agranovsky O., McKinney M. D., Murty V. V., Bauchwitz R. Friend murine leukemia virus-immortalized myeloid cells are converted into tumorigenic cell lines by Abelson leukemia virus. Proc Natl Acad Sci U S A. 1985 May;82(10):3306–3310. doi: 10.1073/pnas.82.10.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H., Siegel J. P., Erdos M., Gnarra J. R., Toledano M. B., Sharon M., Mostowski H., Feinberg M. B., Pierce J. H., Leonard W. J. Interleukin (IL)-2 and IL-3 induce distinct but overlapping responses in murine IL-3-dependent 32D cells transduced with human IL-2 receptor beta chain: involvement of tyrosine kinase(s) other than p56lck. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2789–2793. doi: 10.1073/pnas.89.7.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989 May 19;57(4):521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Quelle D. E., Wojchowski D. M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Brightman K., Scott A., Ihle J. N. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. doi: 10.1038/317434a0. [DOI] [PubMed] [Google Scholar]
- Rein A., Keller J., Schultz A. M., Holmes K. L., Medicus R., Ihle J. N. Infection of immune mast cells by Harvey sarcoma virus: immortalization without loss of requirement for interleukin-3. Mol Cell Biol. 1985 Sep;5(9):2257–2264. doi: 10.1128/mcb.5.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Metcalf D. Induction of macrophage colony-stimulating factor-dependent growth and differentiation after introduction of the murine c-fms gene into FDC-P1 cells. Mol Cell Biol. 1989 Nov;9(11):5081–5092. doi: 10.1128/mcb.9.11.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruscetti S., Aurigemma R., Yuan C. C., Sawyer S., Blair D. G. Induction of erythropoietin responsiveness in murine hematopoietic cells by the gag-myb-ets-containing ME26 virus. J Virol. 1992 Jan;66(1):20–26. doi: 10.1128/jvi.66.1.20-26.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Kuhl W., West C., Beutler E. Complete correction of the enzymatic defect of type I Gaucher disease fibroblasts by retroviral-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Feb;84(4):906–909. doi: 10.1073/pnas.84.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Wright D., Erdman V. D., Cutting A. E. Amphotropic retrovirus vector system for human cell gene transfer. Mol Cell Biol. 1984 Sep;4(9):1730–1737. doi: 10.1128/mcb.4.9.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki S., Tominaga A., Hitoshi Y., Mita S., Sonoda E., Yamaguchi N., Takatsu K. Molecular cloning and expression of the murine interleukin-5 receptor. EMBO J. 1990 Dec;9(13):4367–4374. doi: 10.1002/j.1460-2075.1990.tb07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtieri M., Tweardy D. J., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., Rovera G. Cytokine-dependent granulocytic differentiation. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J Immunol. 1987 Jun 1;138(11):3829–3835. [PubMed] [Google Scholar]
- Wang B. C., Kennan W. S., Yasukawa-Barnes J., Lindstrom M. J., Gould M. N. Carcinoma induction following direct in situ transfer of v-Ha-ras into rat mammary epithelial cells using replication-defective retrovirus vectors. Cancer Res. 1991 May 15;51(10):2642–2648. [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Dunbar C. E., Bodine D. M., Ruscetti S., Nienhuis A. W. Retrovirus-mediated transfer and expression of the interleukin-3 gene in mouse hematopoietic cells result in a myeloproliferative disorder. Mol Cell Biol. 1989 Feb;9(2):798–808. doi: 10.1128/mcb.9.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Nienhuis A. W. Retroviral transfer and expression of the interleukin-3 gene in hemopoietic cells. Genes Dev. 1987 Jun;1(4):358–365. doi: 10.1101/gad.1.4.358. [DOI] [PubMed] [Google Scholar]
- Wongsasant B., Matsuda S., Yamamoto T. Active c-erbB-2 induces short-term growth of FDC-P2 cells after IL-3 depletion. Biochem Biophys Res Commun. 1991 Dec 31;181(3):981–988. doi: 10.1016/0006-291x(91)92033-g. [DOI] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]




