Abstract
The simian immunodeficiency virus SIVsmmPBj14 (SIV-PBj14) is an atypical lentivirus that causes acute disease and death in pig-tailed macaques and in vitro replicates efficiently in resting macaque lymphocytes and activates and induces proliferation of lymphocytes. The present study was conducted to test the hypothesis that production of large quantities of SIV-PBj14 induces widespread immune activation and elaboration of cytokines which lead directly to the death of infected pig-tailed macaques. Following intravenous inoculation of pig-tailed macaques with SIV-PBj14, acute disease developed and was characterized by high levels of plasma viremia, p27gag antigenemia, tumor necrosis factor alpha, and interleukin-6 (IL-6). All animals died within 10 days of infection, at which time some animals had as many as 100% CD4+ cells in the periphery and lymphoid tissues infected. During the last few days before death, titers of infectious virus in blood increased as much as 10(5)-fold. By using dual-label immunofluorescence assays for detection of cell surface activation markers, both CD4+ and CD8+ lymphocytes were shown to express the IL-2 and transferrin receptors following either in vivo or in vitro infection with SIV-PBj14. Furthermore, in vitro infection of quiescent macaque lymphocytes by SIV-PBj14 was accompanied by proliferation of both CD4+ and CD8+ lymphocyte subsets, as measured by incorporation of [3H]thymidine. Increases in numbers of activated lymphocytes and levels of proinflammatory cytokines in plasma coincided with increased amounts of detectable virus in vivo. Clinical signs of disease and pathologic findings were most consistent with death from a shock-like syndrome, in which acute-phase inflammatory cytokines are known to play a major role. Tumor necrosis factor alpha, IL-2, and IL-6 were detected in some cultures infected with SIV-PBj14, but this finding was not consistent. When cytokines were detected, their concentrations were essentially no different from those found in control cultures infected with SIVsmm9, a prototypic strain from which SIV-PBj14 was derived. The in vivo results suggest a synergistic cycle of activation of lymphocytes and monocytes, elaboration of cytokines, and virus production that accelerates uncontrolled and culminates in death. The observed correlations between in vivo and in vitro activation events following SIV-PBj14 infection validate the use of in vitro studies to clarify lentivirus-lymphocyte interactions that may contribute to the virulence of SIV-PBj14.
Full text
PDF

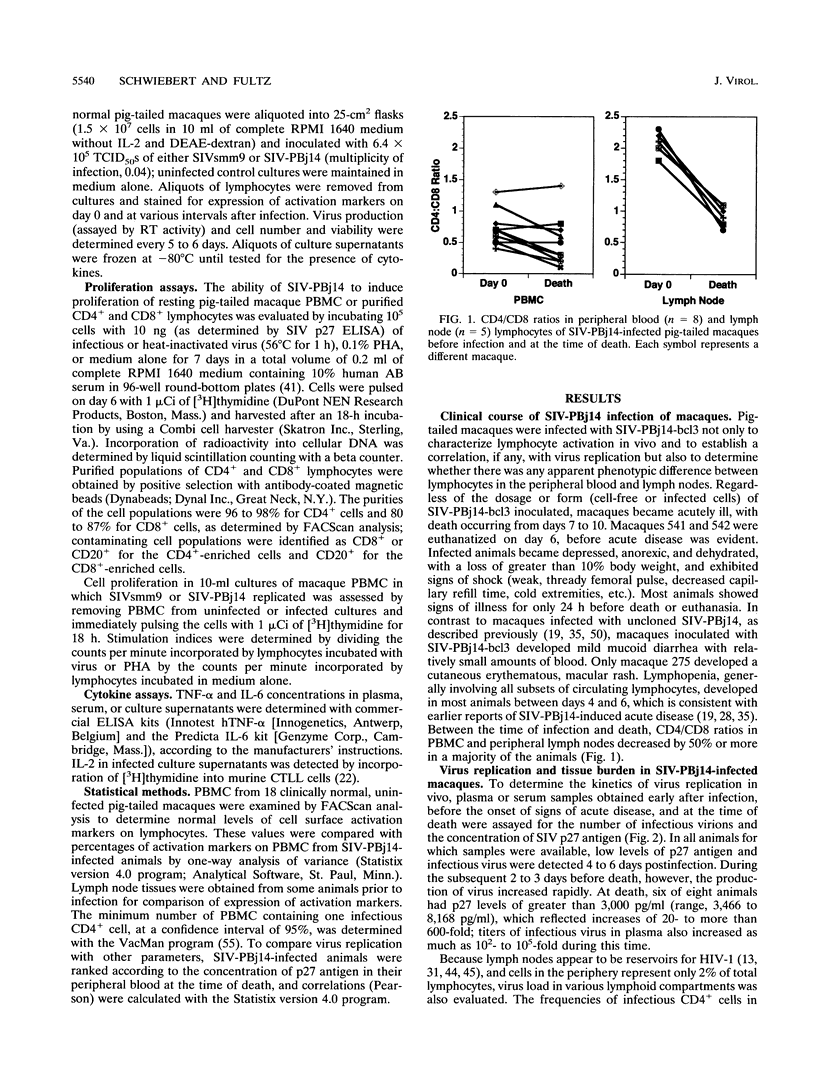
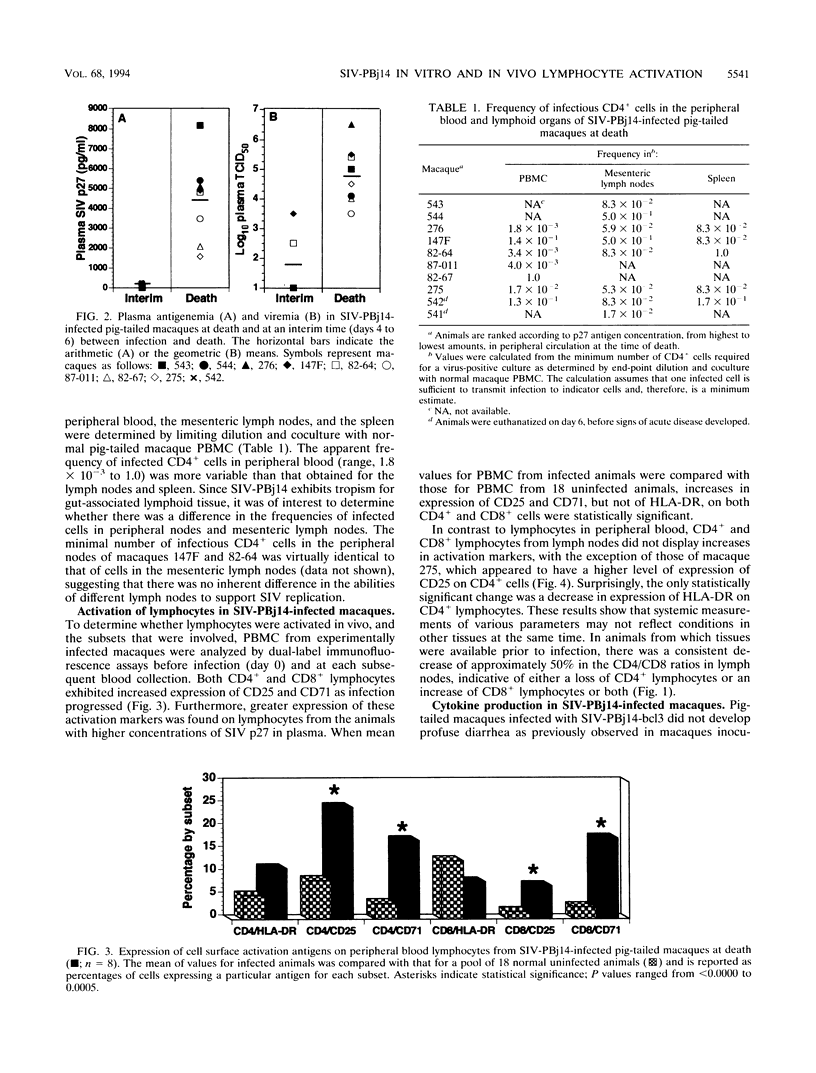
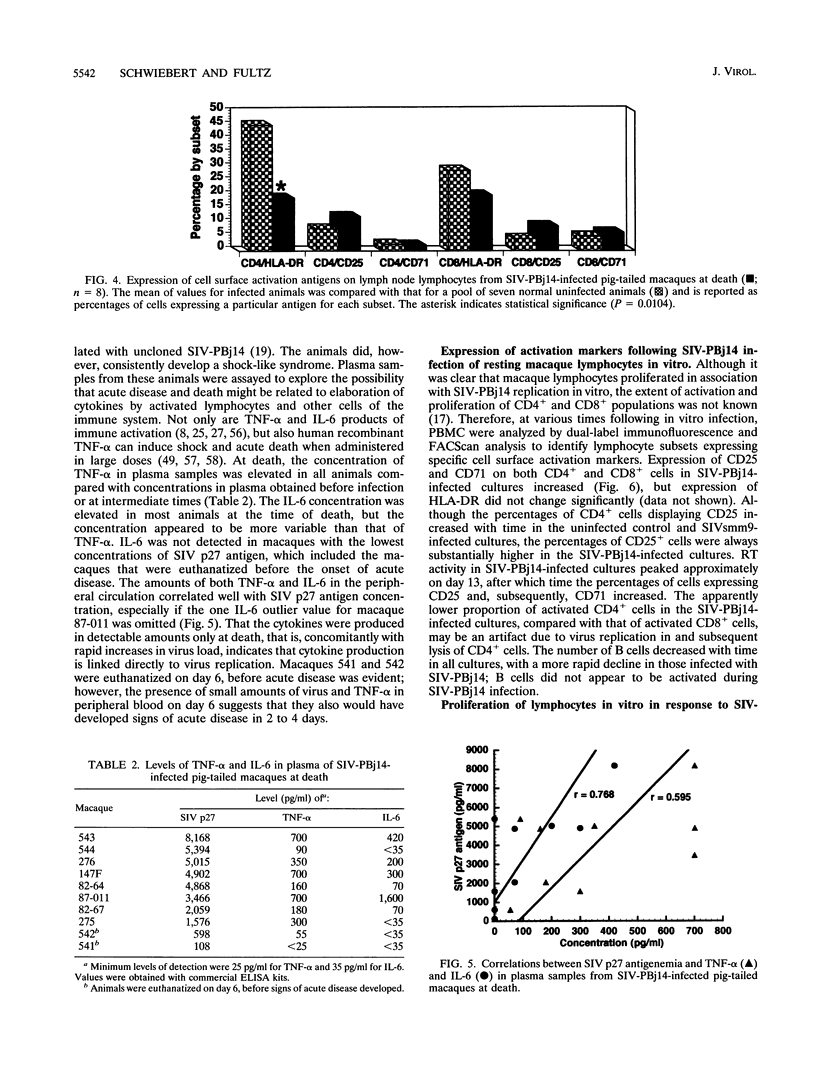
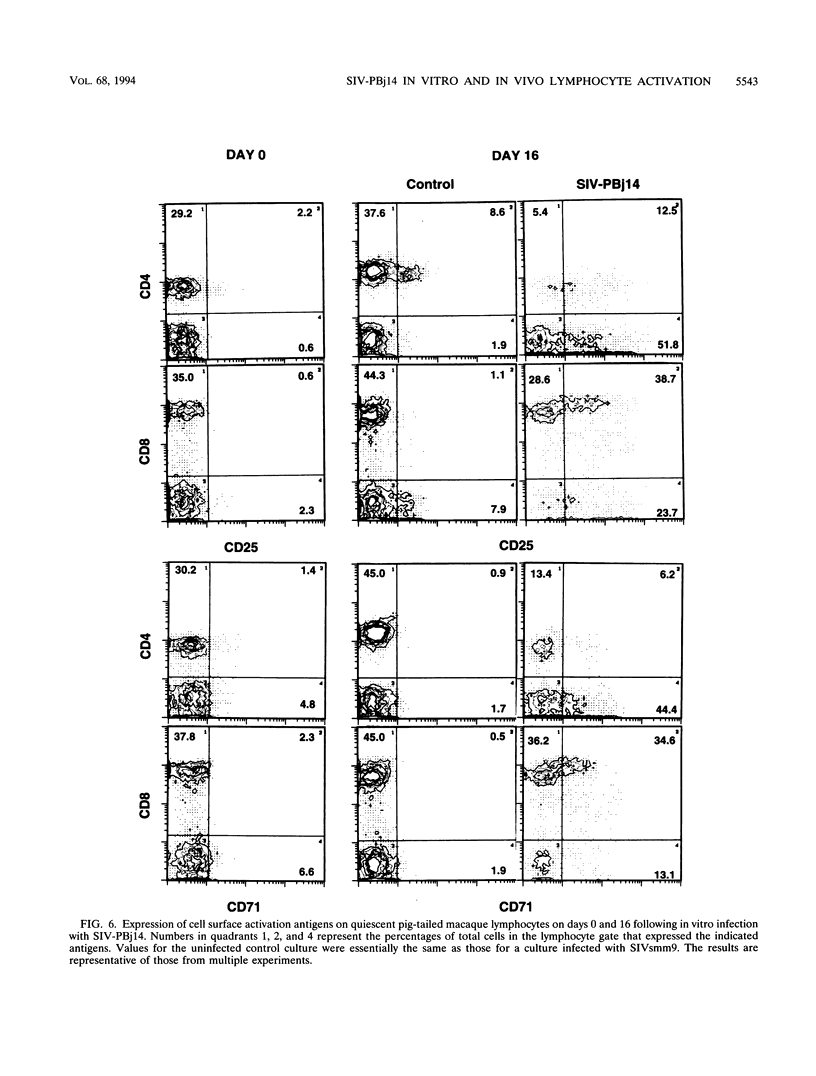



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amadori A., De Rossi A., Gallo P., Tavolato B., Chieco-Bianchi L. Cerebrospinal fluid lymphocytes from HIV-infected patients synthesize HIV-specific antibody in vitro. J Neuroimmunol. 1988 May;18(2):181–186. doi: 10.1016/0165-5728(88)90065-3. [DOI] [PubMed] [Google Scholar]
- Birx D. L., Lewis M. G., Vahey M., Tencer K., Zack P. M., Brown C. R., Jahrling P. B., Tosato G., Burke D., Redfield R. Association of interleukin-6 in the pathogenesis of acutely fatal SIVsmm/PBj-14 in pigtailed macaques. AIDS Res Hum Retroviruses. 1993 Nov;9(11):1123–1129. doi: 10.1089/aid.1993.9.1123. [DOI] [PubMed] [Google Scholar]
- Birx D. L., Redfield R. R., Tencer K., Fowler A., Burke D. S., Tosato G. Induction of interleukin-6 during human immunodeficiency virus infection. Blood. 1990 Dec 1;76(11):2303–2310. [PubMed] [Google Scholar]
- Breen E. C., Rezai A. R., Nakajima K., Beall G. N., Mitsuyasu R. T., Hirano T., Kishimoto T., Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990 Jan 15;144(2):480–484. [PubMed] [Google Scholar]
- Clark S. J., Saag M. S., Decker W. D., Campbell-Hill S., Roberson J. L., Veldkamp P. J., Kappes J. C., Hahn B. H., Shaw G. M. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991 Apr 4;324(14):954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- Cooper D. A., Gold J., Maclean P., Donovan B., Finlayson R., Barnes T. G., Michelmore H. M., Brooke P., Penny R. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet. 1985 Mar 9;1(8428):537–540. doi: 10.1016/s0140-6736(85)91205-x. [DOI] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Hunsmann G., Schmidt D. K., King N. W., Desrosiers R. C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987 Dec;68(Pt 12):3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Debets J. M., Kampmeijer R., van der Linden M. P., Buurman W. A., van der Linden C. J. Plasma tumor necrosis factor and mortality in critically ill septic patients. Crit Care Med. 1989 Jun;17(6):489–494. doi: 10.1097/00003246-198906000-00001. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Embretson J. E., Anderson D. C., Mullins J. I., Fultz P. N. Sequence analysis and acute pathogenicity of molecularly cloned SIVSMM-PBj14. Nature. 1990 Jun 14;345(6276):636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993 Nov 12;262(5136):1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Switzer W. M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res Hum Retroviruses. 1989 Aug;5(4):397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- Fultz P. N. Replication of an acutely lethal simian immunodeficiency virus activates and induces proliferation of lymphocytes. J Virol. 1991 Sep;65(9):4902–4909. doi: 10.1128/jvi.65.9.4902-4909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N., Zack P. M. Unique lentivirus--host interactions: SIVsmmPBj14 infection of macaques. Virus Res. 1994 May;32(2):205–225. doi: 10.1016/0168-1702(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Gallo P., Frei K., Rordorf C., Lazdins J., Tavolato B., Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989 Jul;23(2):109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Green E. M., Adams H. R. New perspectives in circulatory shock: pathophysiologic mediators of the mammalian response to endotoxemia and sepsis. J Am Vet Med Assoc. 1992 Jun 15;200(12):1834–1841. [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Resnick L., Dimarzoveronese F., Rota T. R., Hirsch M. S. Primary human T-lymphotropic virus type III infection. Ann Intern Med. 1985 Dec;103(6 ):880–883. doi: 10.7326/0003-4819-103-6-880. [DOI] [PubMed] [Google Scholar]
- Holsti M. A., Raulet D. H. IL-6 and IL-1 synergize to stimulate IL-2 production and proliferation of peripheral T cells. J Immunol. 1989 Oct 15;143(8):2514–2519. [PubMed] [Google Scholar]
- Honda M., Kitamura K., Mizutani Y., Oishi M., Arai M., Okura T., Igarahi K., Yasukawa K., Hirano T., Kishimoto T. Quantitative analysis of serum IL-6 and its correlation with increased levels of serum IL-2R in HIV-induced diseases. J Immunol. 1990 Dec 15;145(12):4059–4064. [PubMed] [Google Scholar]
- Houssiau F. A., Coulie P. G., Olive D., Van Snick J. Synergistic activation of human T cells by interleukin 1 and interleukin 6. Eur J Immunol. 1988 Apr;18(4):653–656. doi: 10.1002/eji.1830180427. [DOI] [PubMed] [Google Scholar]
- Israel Z. R., Dean G. A., Maul D. H., O'Neil S. P., Dreitz M. J., Mullins J. I., Fultz P. N., Hoover E. A. Early pathogenesis of disease caused by SIVsmmPBj14 molecular clone 1.9 in macaques. AIDS Res Hum Retroviruses. 1993 Mar;9(3):277–286. doi: 10.1089/aid.1993.9.277. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeuillade A., Tamalet C., Pellegrino P., Tourres C., Yahi N., Vignoli C., Quilichini R., de Micco P. High viral burden in lymph nodes during early stages of HIV-1 infection. AIDS. 1993 Nov;7(11):1527–1528. doi: 10.1097/00002030-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., King N. W. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3(11):1023–1040. [PubMed] [Google Scholar]
- Lewis M. G., Zack P. M., Elkins W. R., Jahrling P. B. Infection of rhesus and cynomolgus macaques with a rapidly fatal SIV (SIVSMM/PBj) isolate from sooty mangabeys. AIDS Res Hum Retroviruses. 1992 Sep;8(9):1631–1639. doi: 10.1089/aid.1992.8.1631. [DOI] [PubMed] [Google Scholar]
- Lähdevirta J., Maury C. P., Teppo A. M., Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988 Sep;85(3):289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- McClure H. M., Anderson D. C., Fultz P. N., Ansari A. A., Lockwood E., Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989 May;21(1):13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- McEntee M. F., Gorrell M. D., Adams R. J., Narayan O., Pitha P. Tumour necrosis factor and interleukin 6 production during interaction between activated CD4+ lymphocytes and simian immunodeficiency virus-infected macrophages. J Gen Virol. 1992 May;73(Pt 5):1107–1113. doi: 10.1099/0022-1317-73-5-1107. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Griffith B. P., Ortiz M. A., Landry M. L., Ryan J. L. Tumor necrosis factor-alpha/cachectin enhances human immunodeficiency virus type 1 replication in primary macrophages. J Infect Dis. 1991 Jan;163(1):78–82. doi: 10.1093/infdis/163.1.78. [DOI] [PubMed] [Google Scholar]
- Mintz M., Rapaport R., Oleske J. M., Connor E. M., Koenigsberger M. R., Denny T., Epstein L. G. Elevated serum levels of tumor necrosis factor are associated with progressive encephalopathy in children with acquired immunodeficiency syndrome. Am J Dis Child. 1989 Jul;143(7):771–774. doi: 10.1001/archpedi.1989.02150190021012. [DOI] [PubMed] [Google Scholar]
- Niu M. T., Stein D. S., Schnittman S. M. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis. 1993 Dec;168(6):1490–1501. doi: 10.1093/infdis/168.6.1490. [DOI] [PubMed] [Google Scholar]
- Novembre F. J., Johnson P. R., Lewis M. G., Anderson D. C., Klumpp S., McClure H. M., Hirsch V. M. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J Virol. 1993 May;67(5):2466–2474. doi: 10.1128/jvi.67.5.2466-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K., Nakano T., Matsumoto Y., Watari T., Goitsuka R., Nakayama H., Tsujimoto H., Hasegawa A. Apoptosis induced by tumor necrosis factor in cells chronically infected with feline immunodeficiency virus. J Virol. 1993 May;67(5):2429–2433. doi: 10.1128/jvi.67.5.2429-2433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P. A., Schnittman S. M., Kotler D. P., Fauci A. S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Poli G., Bressler P., Kinter A., Duh E., Timmer W. C., Rabson A., Justement J. S., Stanley S., Fauci A. S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990 Jul 1;172(1):151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkonen P., Kaaya E. E., Böttiger D., Li S. L., Nilsson C., Biberfeld P., Biberfeld G. Clinical features and predictive markers of disease progression in cynomolgus monkeys experimentally infected with simian immunodeficiency virus. AIDS. 1992 Mar;6(3):257–263. doi: 10.1097/00002030-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick D. G., Kunkel R. G., Larrick J. W., Kunkel S. L. Acute in vivo effects of human recombinant tumor necrosis factor. Lab Invest. 1987 Jun;56(6):583–590. [PubMed] [Google Scholar]
- Rosenberg Y. J., White B. D., Papermaster S. F., Zack P., Jarling P. B., Eddy G. A., Burke D. S., Lewis M. G. Variation in T-lymphocyte activation and susceptibility to SIVPBj-14-induced acute death in macaques. J Med Primatol. 1991 Jun;20(4):206–210. [PubMed] [Google Scholar]
- Rosenberg Y. J., Zack P. M., White B. D., Papermaster S. F., Elkins W. R., Eddy G. A., Lewis M. G. Decline in the CD4+ lymphocyte population in the blood of SIV-infected macaques is not reflected in lymph nodes. AIDS Res Hum Retroviruses. 1993 Jul;9(7):639–646. doi: 10.1089/aid.1993.9.639. [DOI] [PubMed] [Google Scholar]
- Ryffel B., Kammüller M., Robison R., Myers L. Pathology induced by interleukin-6. Toxicol Lett. 1992 Dec;64-65 Spec No:311–319. doi: 10.1016/0378-4274(92)90203-v. [DOI] [PubMed] [Google Scholar]
- Sinicco A., Biglino A., Sciandra M., Forno B., Pollono A. M., Raiteri R., Gioannini P. Cytokine network and acute primary HIV-1 infection. AIDS. 1993 Sep;7(9):1167–1172. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Spira T. J., Bozeman L. H., Holman R. C., Warfield D. T., Phillips S. K., Feorino P. M. Micromethod for assaying reverse transcriptase of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. J Clin Microbiol. 1987 Jan;25(1):97–99. doi: 10.1128/jcm.25.1.97-99.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spouge J. L. Statistical analysis of sparse infection data and its implications for retroviral treatment trials in primates. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7581–7585. doi: 10.1073/pnas.89.16.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Bjorndahl J. M., Wang C. Y., Kao H. T., Fu S. M. Production of tumor necrosis factor/cachectin by human T cell lines and peripheral blood T lymphocytes stimulated by phorbol myristate acetate and anti-CD3 antibody. J Exp Med. 1988 Mar 1;167(3):937–953. doi: 10.1084/jem.167.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Vyakarnam A., McKeating J., Meager A., Beverley P. C. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS. 1990 Jan;4(1):21–27. doi: 10.1097/00002030-199001000-00003. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Walsh D. G., Horvath C. J., Hansen-Moosa A., MacKey J. J., Sehgal P. K., Daniel M. D., Desrosiers R. C., Ringler D. J. Cytokine influence on simian immunodeficiency virus replication within primary macrophages. TNF-alpha, but not GMCSF, enhances viral replication on a per-cell basis. Am J Pathol. 1991 Oct;139(4):877–887. [PMC free article] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]


