Abstract
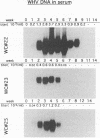
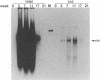
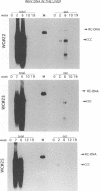
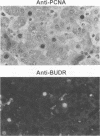
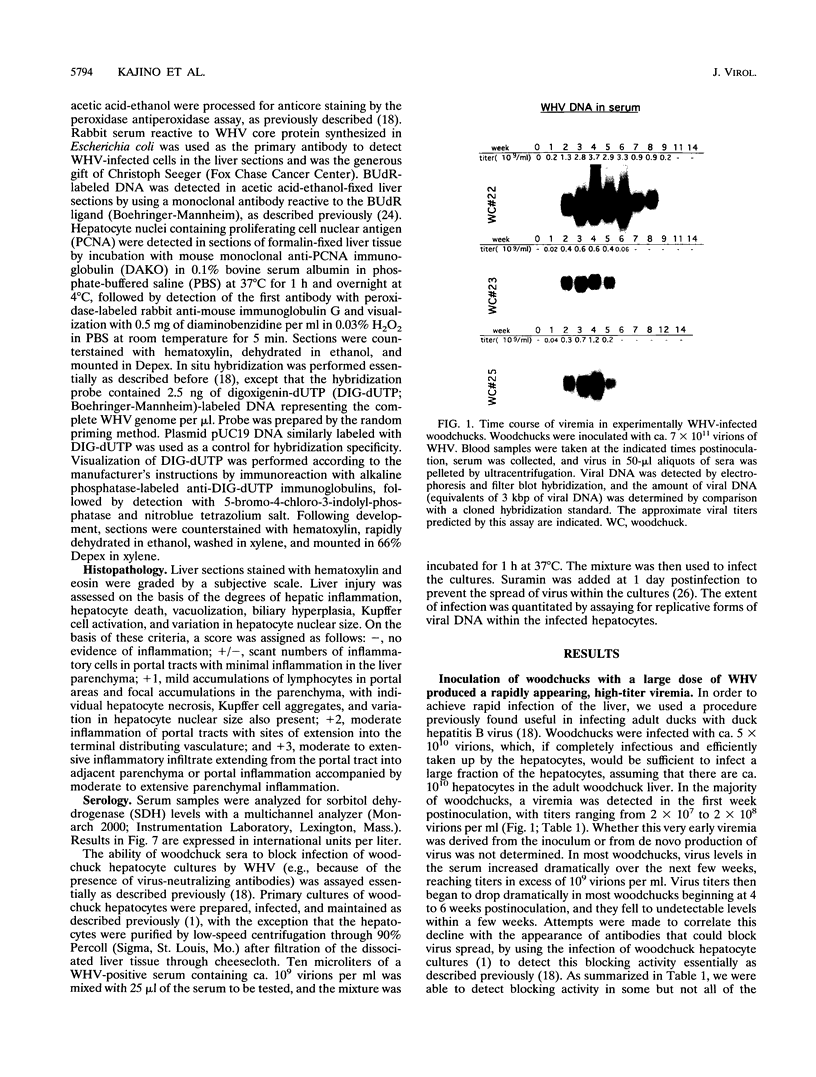
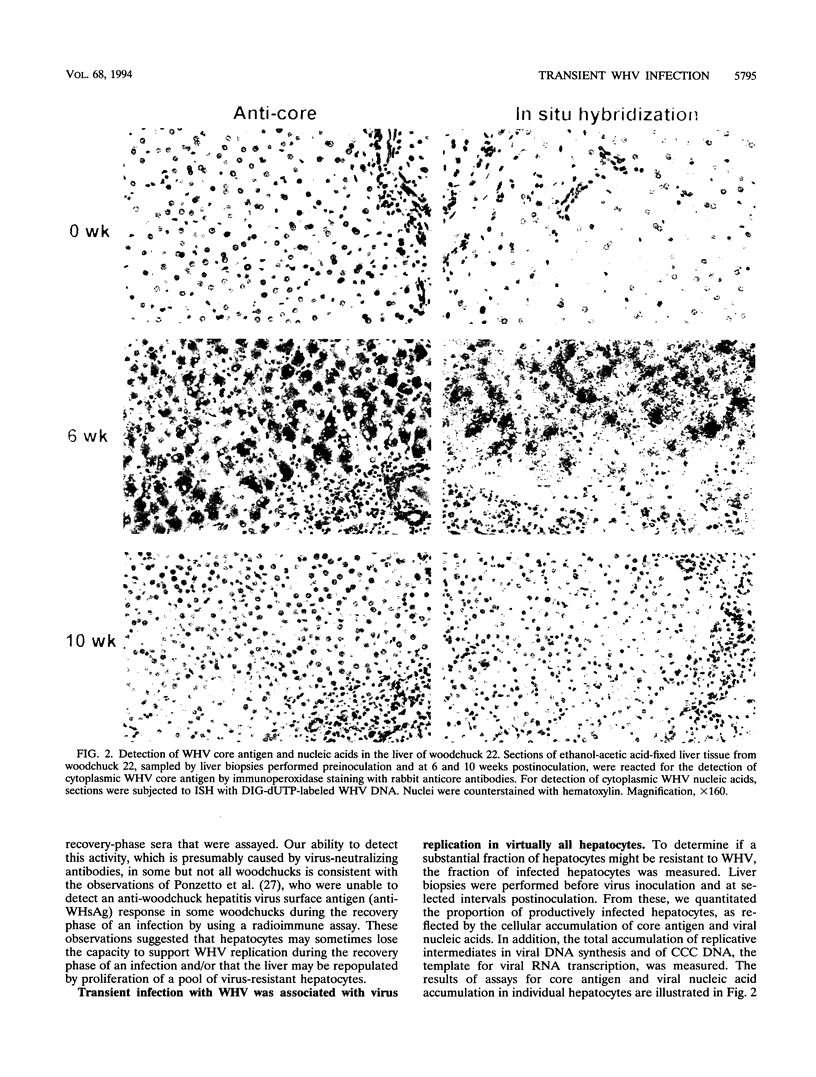
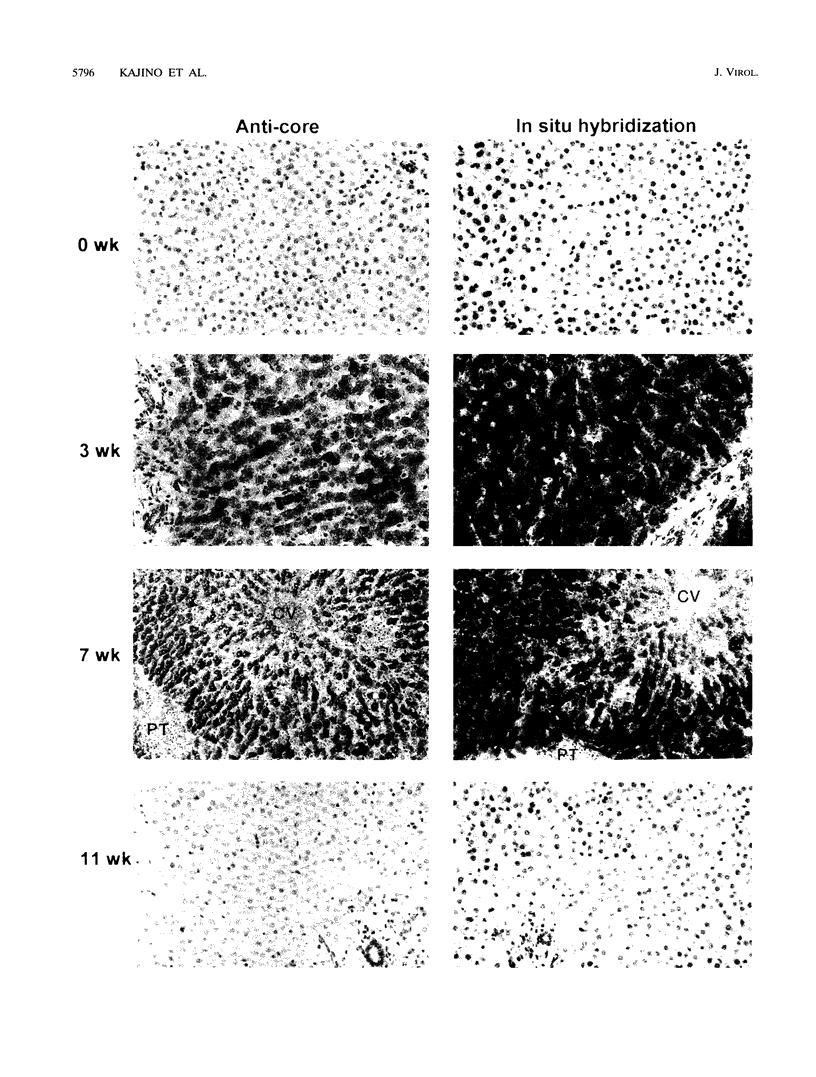
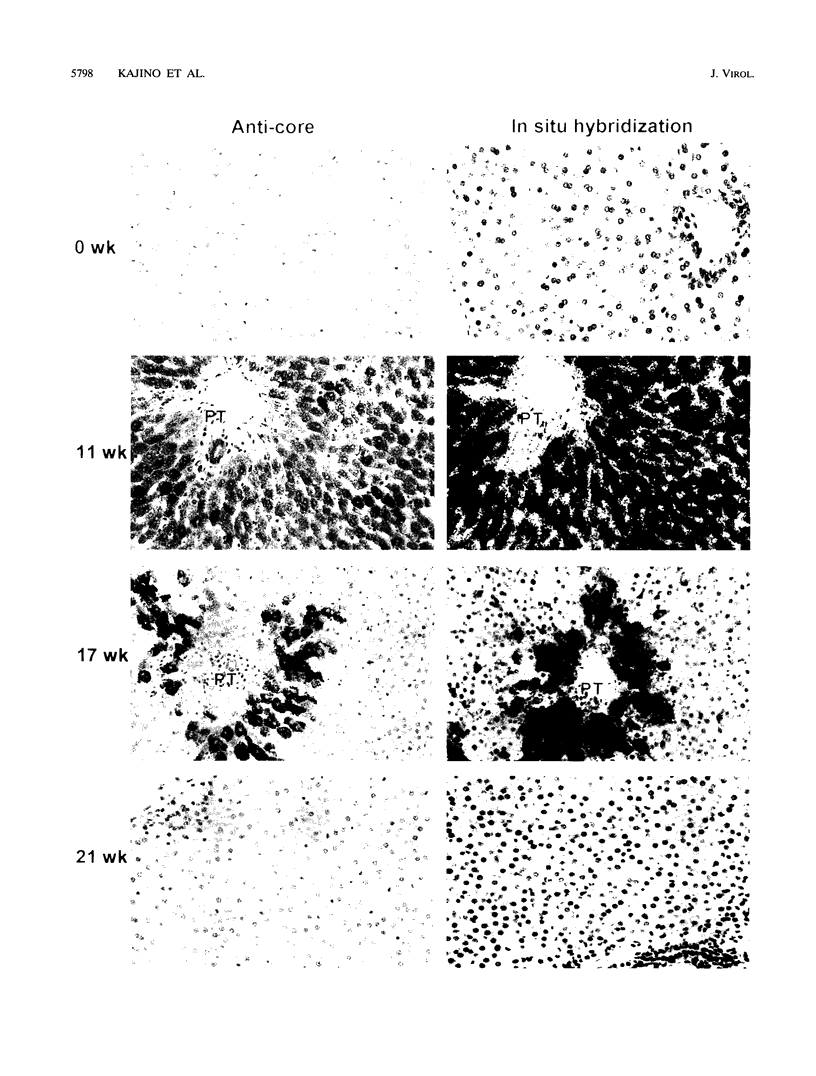
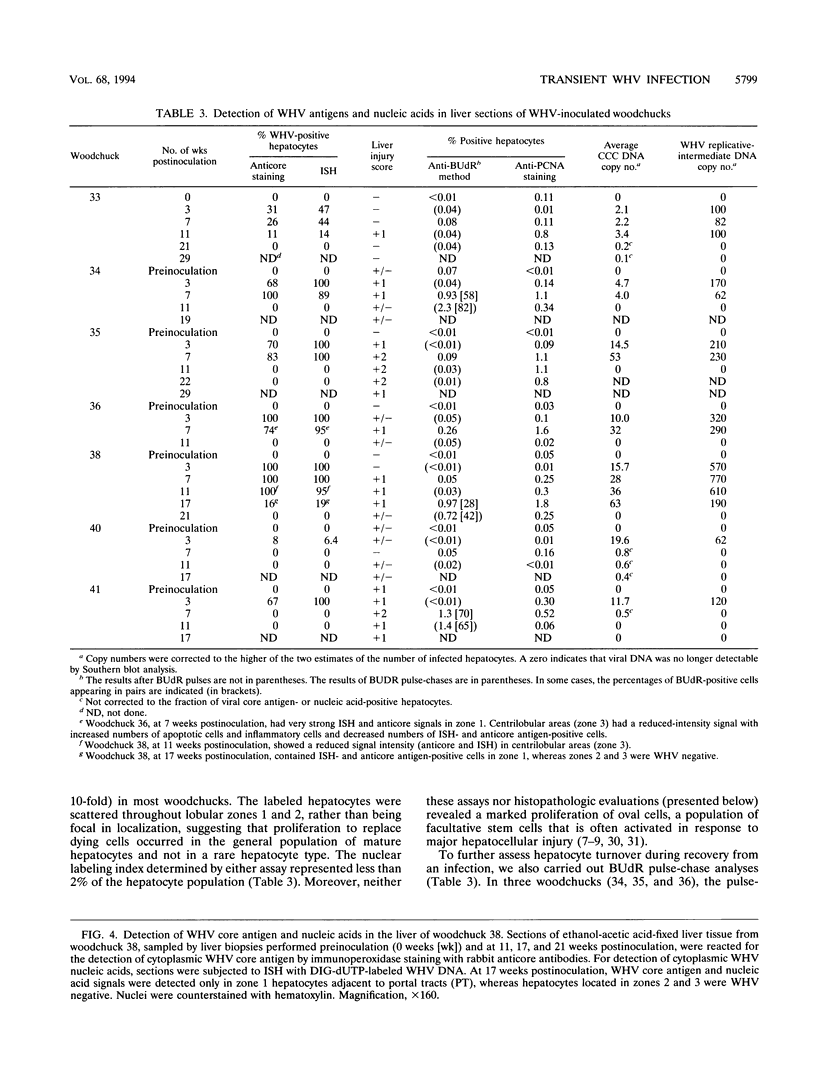
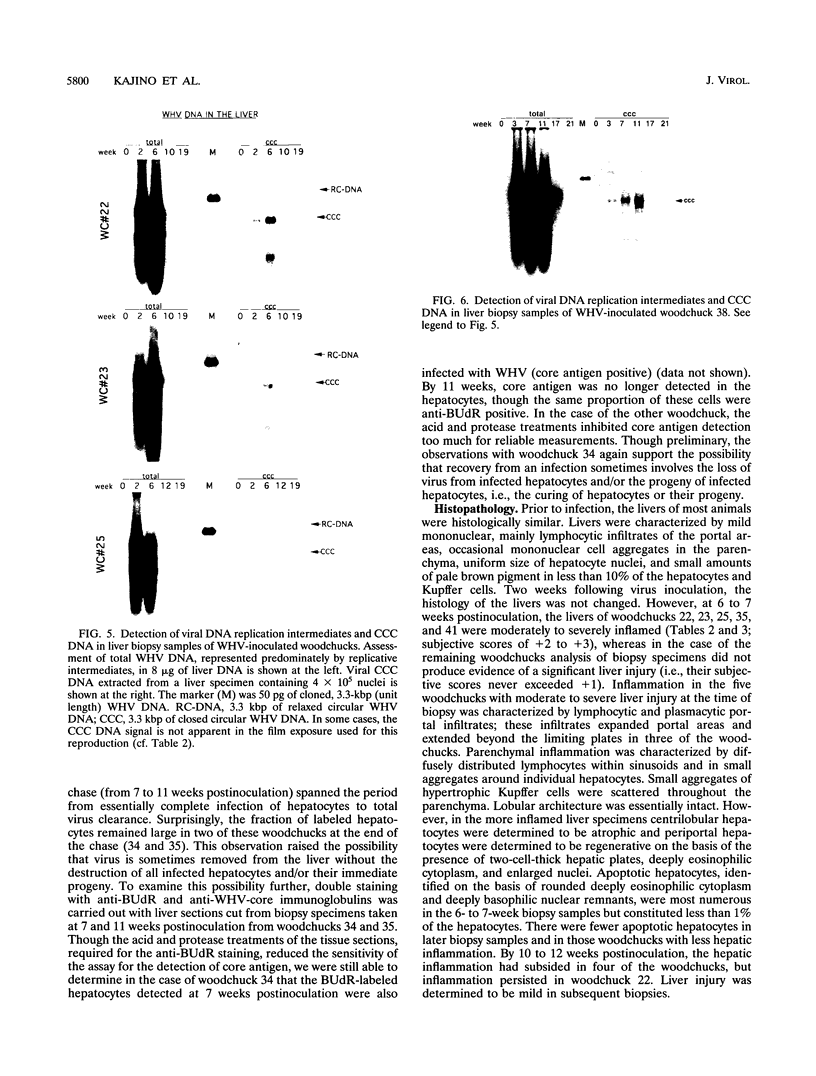
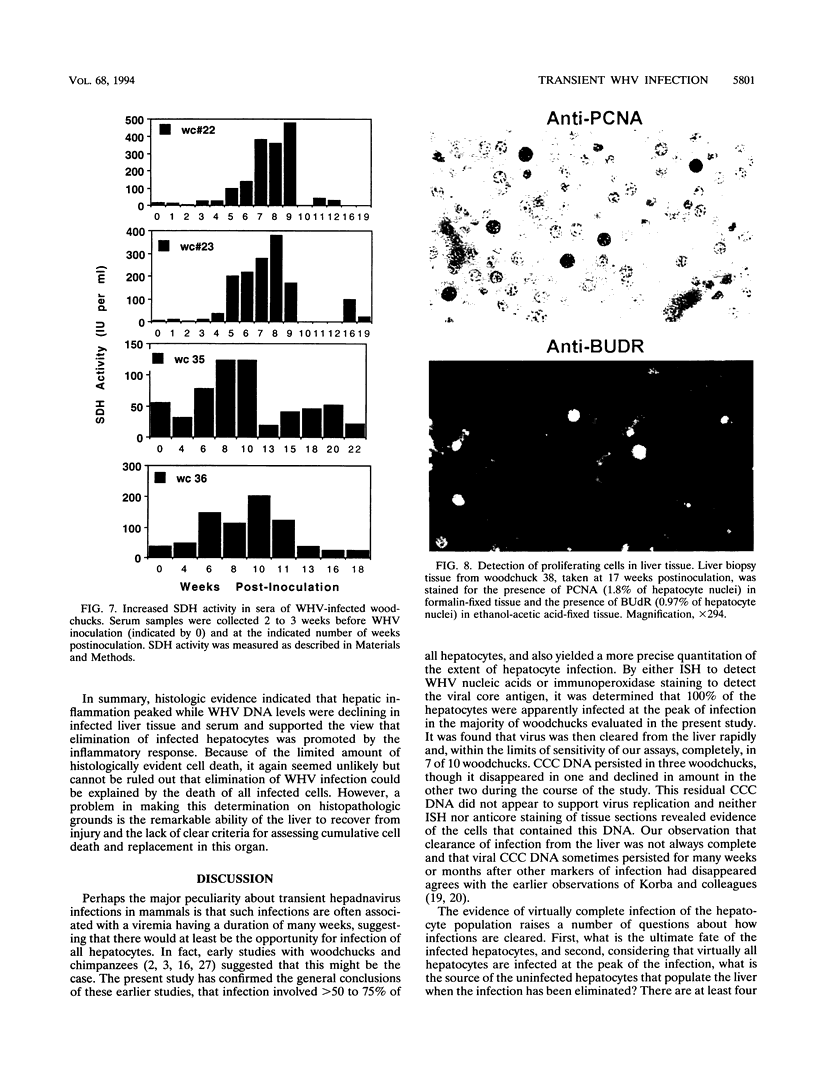
Earlier studies have suggested that transient hepadnavirus infections in mammals are associated with virus replication in a large fraction of hepatocytes. Although the viremia that occurred during transient infections in some individuals would presumably lead to virus replication in all hepatocytes, these studies did not reveal if this was the case. The question of the extent of hepatocyte infection was therefore reinvestigated because of the implications of the results for the mechanisms of virus clearance. Woodchucks were inoculated with woodchuck hepatitis virus, and the course of hepatic infection was determined. These studies indicated that essentially 100% of the hepatocytes became infected in the majority of woodchucks. In 7 of 10 woodchucks, the viral infection was then rapidly cleared from the liver, generally in less than 4 weeks. In another three woodchucks, though productive infection was just as rapidly cleared, viral covalently closed circular DNA remained for weeks to months after other indicators of virus infection had disappeared from the liver. Bromodeoxyuridine labeling and anti-proliferating cell nuclear antigen staining to detect hepatocytes passing through S phase indicated an increase in hepatocyte proliferation during the recovery phase of infection. The rate of cell division appeared to be sufficient to replace no more than 2 to 3% of the hepatocytes per day, at the times at which the biopsies were performed. Histopathologic evaluation of the biopsy samples did not provide evidence for a massive amount of liver regeneration. Models to explain virus clearance, with or without massive immune system-mediated destruction of infected hepatocytes, are reviewed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich C. E., Coates L., Wu T. T., Newbold J., Tennant B. C., Summers J., Seeger C., Mason W. S. In vitro infection of woodchuck hepatocytes with woodchuck hepatitis virus and ground squirrel hepatitis virus. Virology. 1989 Sep;172(1):247–252. doi: 10.1016/0042-6822(89)90126-8. [DOI] [PubMed] [Google Scholar]
- Barker L. F., Chisari F. V., McGrath P. P., Dalgard D. W., Kirschstein R. L., Almeida J. D., Edington T. S., Sharp D. G., Peterson M. R. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis. 1973 Jun;127(6):648–662. doi: 10.1093/infdis/127.6.648. [DOI] [PubMed] [Google Scholar]
- Barker L. F., Chisari F. V., McGrath P. P., Dalgard D. W., Kirschstein R. L., Almeida J. D., Edington T. S., Sharp D. G., Peterson M. R. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis. 1973 Jun;127(6):648–662. doi: 10.1093/infdis/127.6.648. [DOI] [PubMed] [Google Scholar]
- Carp N. Z., Saputelli J., Halbherr T. C., Mason W. S., Jilbert A. R. A technique for liver biopsy performed in Pekin ducks using anesthesia with Telazol. Lab Anim Sci. 1991 Oct;41(5):474–475. [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Dabeva M. D., Alpini G., Hurston E., Shafritz D. A. Models for hepatic progenitor cell activation. Proc Soc Exp Biol Med. 1993 Dec;204(3):242–252. doi: 10.3181/00379727-204-43660. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Fausto N., Lemire J. M., Shiojiri N. Cell lineages in hepatic development and the identification of progenitor cells in normal and injured liver. Proc Soc Exp Biol Med. 1993 Dec;204(3):237–241. doi: 10.3181/00379727-204-43659. [DOI] [PubMed] [Google Scholar]
- Frommel D., Crevat D., Vitvitsky L., Pichoud C., Hantz O., Chevalier M., Grimaud J. A., Lindberg J., Trépo C. G. Immunopathologic aspects of woodchuck hepatitis. Am J Pathol. 1984 Apr;115(1):125–134. [PMC free article] [PubMed] [Google Scholar]
- Galand P., Degraef C. Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labelling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet. 1989 Sep;22(5):383–392. doi: 10.1111/j.1365-2184.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Gilles P. N., Fey G., Chisari F. V. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J Virol. 1992 Jun;66(6):3955–3960. doi: 10.1128/jvi.66.6.3955-3960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L. G., Ando K., Hobbs M. V., Ishikawa T., Runkel L., Schreiber R. D., Chisari F. V. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L. G., Guilhot S., Chisari F. V. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J Virol. 1994 Mar;68(3):1265–1270. doi: 10.1128/jvi.68.3.1265-1270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhot S., Guidotti L. G., Chisari F. V. Interleukin-2 downregulates hepatitis B virus gene expression in transgenic mice by a posttranscriptional mechanism. J Virol. 1993 Dec;67(12):7444–7449. doi: 10.1128/jvi.67.12.7444-7449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle J. H., Michalak T., Nowoslawski A., Gerety R. J., Barker L. F. Immunofluorescence microscopy in experimentally induced, type B hepatitis in the chimpanzee. Gastroenterology. 1978 Feb;74(2 Pt 1):182–187. [PubMed] [Google Scholar]
- Hornbuckle W. E., Graham E. S., Roth L., Baldwin B. H., Wickenden C., Tennant B. C. Laboratory assessment of hepatic injury in the woodchuck (Marmota monax). Lab Anim Sci. 1985 Aug;35(4):376–381. [PubMed] [Google Scholar]
- Jilbert A. R., Wu T. T., England J. M., Hall P. M., Carp N. Z., O'Connell A. P., Mason W. S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992 Mar;66(3):1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Cote P. J., Wells F. V., Baldwin B., Popper H., Purcell R. H., Tennant B. C., Gerin J. L. Natural history of woodchuck hepatitis virus infections during the course of experimental viral infection: molecular virologic features of the liver and lymphoid tissues. J Virol. 1989 Mar;63(3):1360–1370. doi: 10.1128/jvi.63.3.1360-1370.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Gowans E. J., Wells F. V., Tennant B. C., Clarke R., Gerin J. L. Systemic distribution of woodchuck hepatitis virus in the tissues of experimentally infected woodchucks. Virology. 1988 Jul;165(1):172–181. doi: 10.1016/0042-6822(88)90670-8. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Wells F. V., Baldwin B., Cote P. J., Tennant B. C., Popper H., Gerin J. L. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology. 1989 Mar;9(3):461–470. doi: 10.1002/hep.1840090321. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Knight S. S., Salazar F. H., Popper H., Robinson W. S. Ground squirrel hepatitis virus infection. Hepatology. 1983 Jul-Aug;3(4):519–527. doi: 10.1002/hep.1840030408. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Cullen J., Saputelli J., Wu T. T., Liu C., London W. T., Lustbader E., Schaffer P., O'Connell A. P., Fourel I. Characterization of the antiviral effects of 2' carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology. 1994 Feb;19(2):398–411. [PubMed] [Google Scholar]
- Millman I., Southam L., Halbherr T., Simmons H., Kang C. M. Woodchuck hepatitis virus: experimental infection and natural occurrence. Hepatology. 1984 Sep-Oct;4(5):817–823. doi: 10.1002/hep.1840040503. [DOI] [PubMed] [Google Scholar]
- Petcu D. J., Aldrich C. E., Coates L., Taylor J. M., Mason W. S. Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology. 1988 Dec;167(2):385–392. [PubMed] [Google Scholar]
- Ponzetto A., Cote P. J., Ford E. C., Purcell R. H., Gerin J. L. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984 Oct;52(1):70–76. doi: 10.1128/jvi.52.1.70-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler C. E. Cellular and molecular mechanisms of hepatocarcinogenesis associated with hepadnavirus infection. Curr Top Microbiol Immunol. 1991;168:103–140. doi: 10.1007/978-3-642-76015-0_6. [DOI] [PubMed] [Google Scholar]
- Sandgren E. P., Palmiter R. D., Heckel J. L., Daugherty C. C., Brinster R. L., Degen J. L. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991 Jul 26;66(2):245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- Sells M. A., Zelent A. Z., Shvartsman M., Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988 Aug;62(8):2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson S. S., Evarts R. P., Bisgaard H. C., Fujio K., Hu Z. Hepatic stem cell compartment: activation and lineage commitment. Proc Soc Exp Biol Med. 1993 Dec;204(3):253–260. doi: 10.3181/00379727-204-43661. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]