Abstract
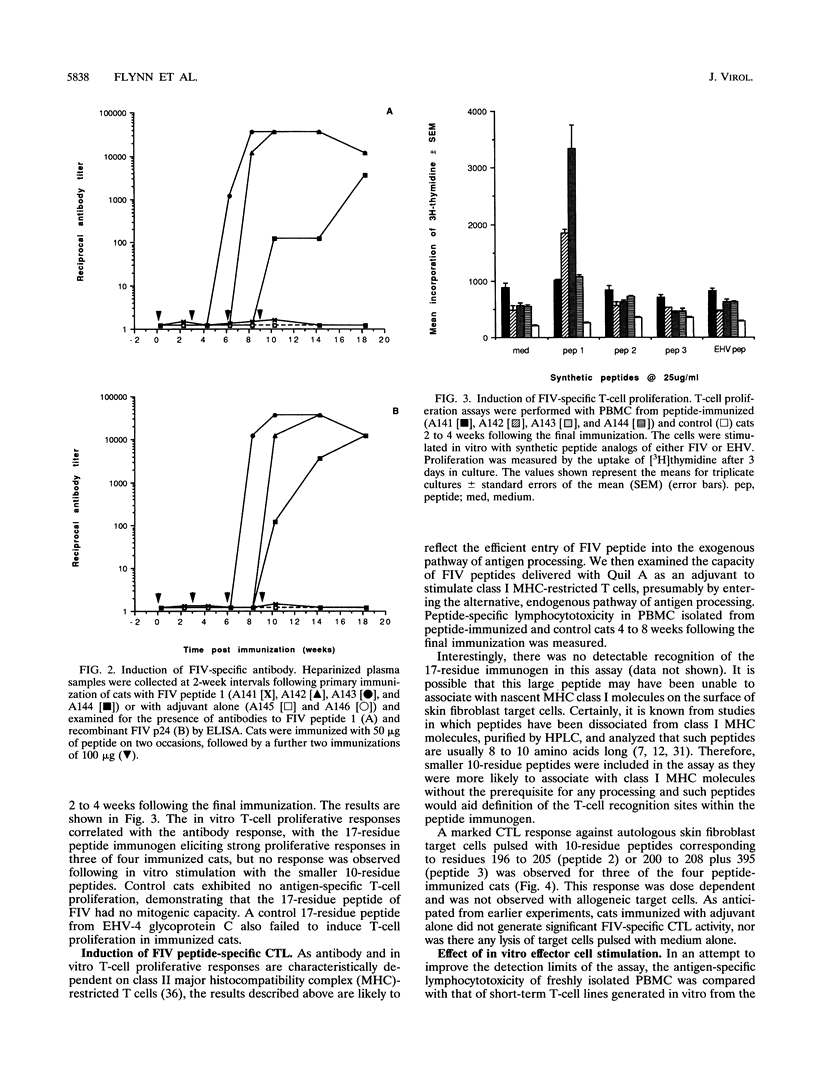
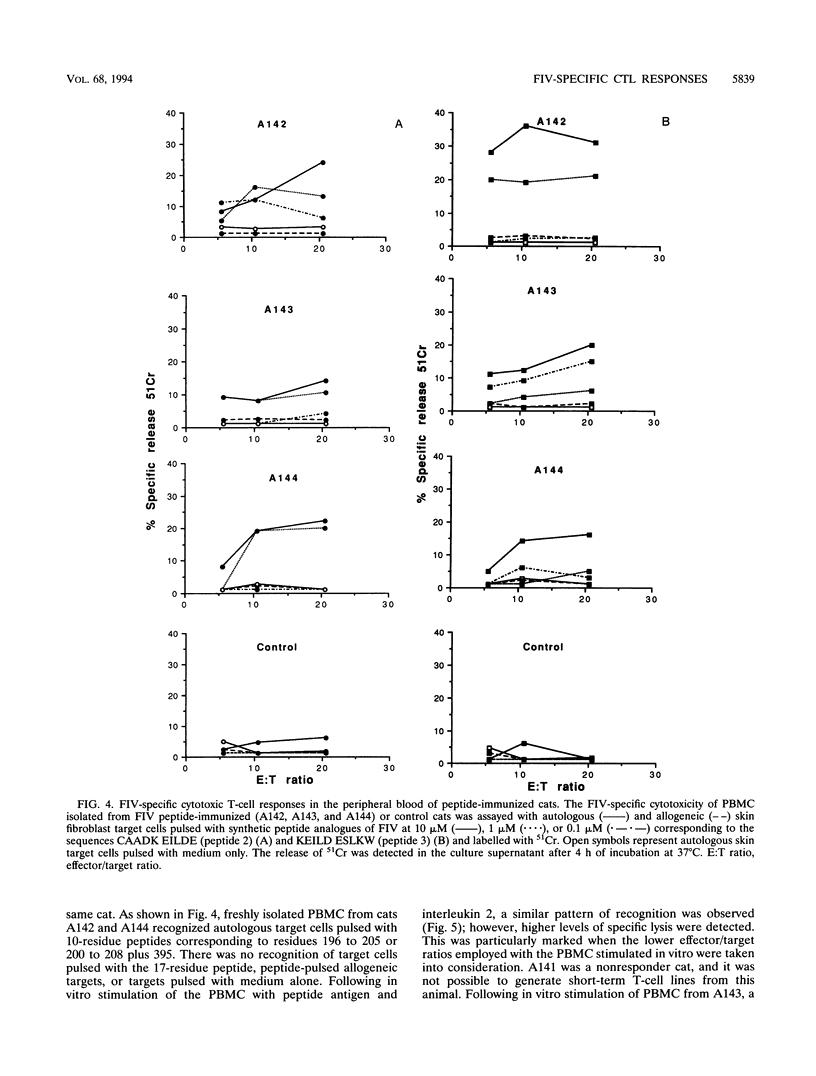
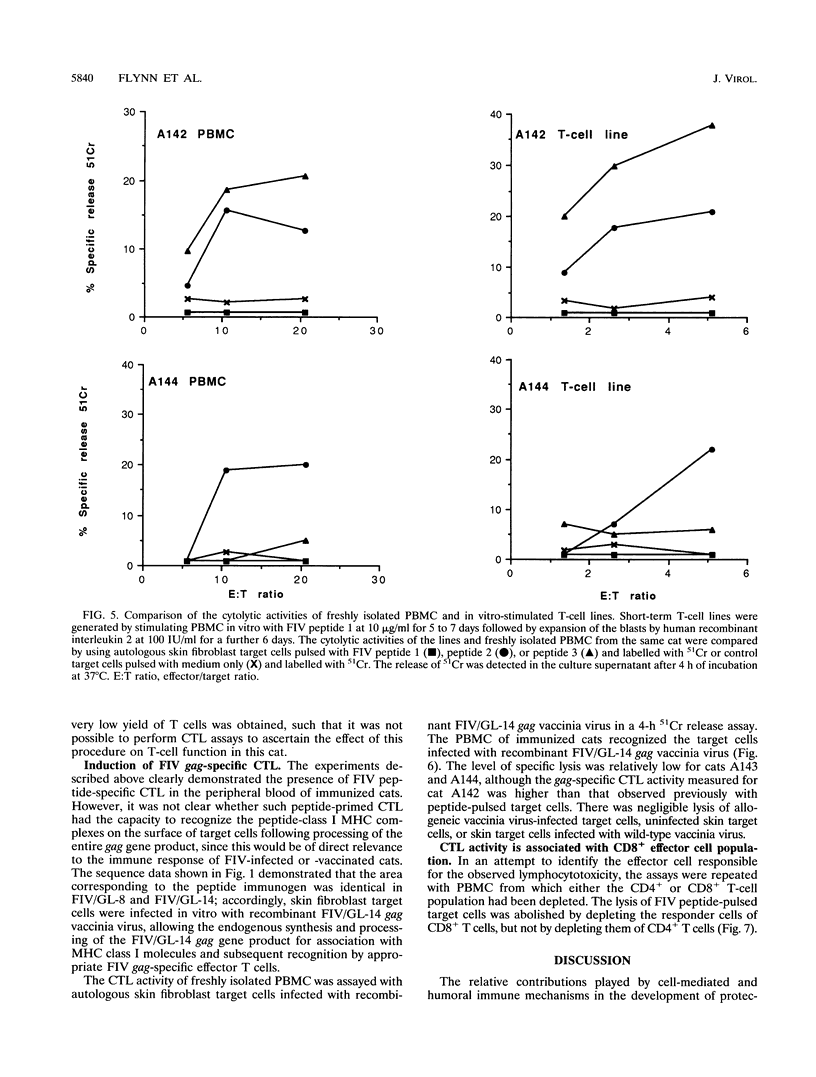
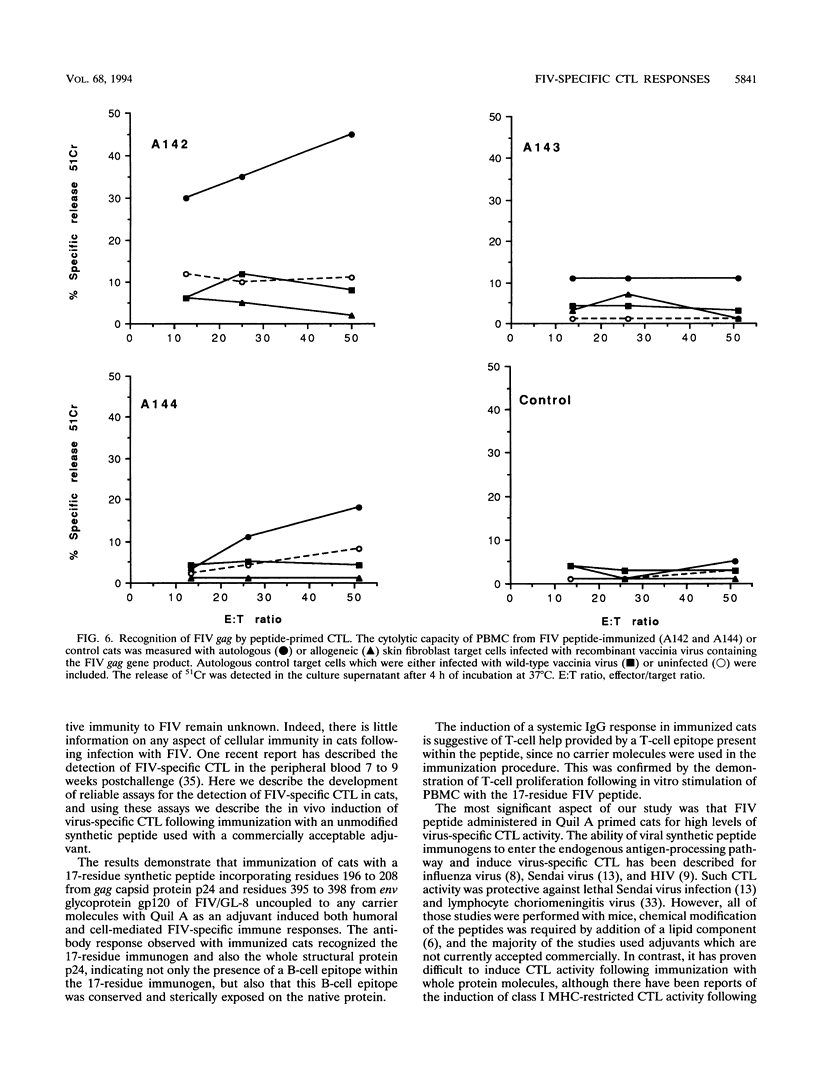
The role of cellular immunity in the establishment and progression of immunosuppressive lentivirus infection remains equivocal. To develop a model system with which these aspects of the host immune response can be studied experimentally, we examined the response of cats to a hybrid peptide containing predicted T-and B-cell epitopes from the gag and env genes of feline immunodeficiency virus (FIV). Cats were immunized with an unmodified 17-residue peptide incorporating residues 196 to 208 (from gag capsid protein p24) and 395 to 398 (from env glycoprotein gp120) of the FIV Glasgow-8 strain by using Quil A as an adjuvant. Virus-specific lymphocytotoxicity was measured by chromium-51 release assays. The target cells were autologous or allogeneic skin fibroblasts either infected with recombinant FIV gag vaccinia virus or pulsed with FIV peptides. Effector cells were either fresh peripheral blood mononuclear cells or T-cell lines stimulated with FIV peptides in vitro. Cytotoxic effector cells from immunized cats lysed autologous, but not allogeneic, target cells when they were either infected with recombinant FIV gag vaccinia virus or pulsed with synthetic peptides comprising residues 196 to 205 or 200 to 208 plus 395. Depletion of CD8+ T cells, from the effector cell population abrogated the lymphocytotoxicity. Immunized cats developed an antibody response to the 17-residue peptide immunogen and to recombinant p24. However, no antibodies which recognized smaller constituent peptides could be detected. This response correlated with peptide-induced T-cell proliferation in vitro. This study demonstrates that cytotoxic T lymphocytes specific for FIV can be induced following immunization with an unmodified short synthetic peptide and defines a system in which the protective or pathological role of such responses can be examined.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aichele P., Hengartner H., Zinkernagel R. M., Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990 May 1;171(5):1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Feinberg M. B. HIV revealed: toward a natural history of the infection. N Engl J Med. 1989 Dec 14;321(24):1673–1675. doi: 10.1056/NEJM198912143212409. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheynier R., Langlade-Demoyen P., Marescot M. R., Blanche S., Blondin G., Wain-Hobson S., Griscelli C., Vilmer E., Plata F. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. Eur J Immunol. 1992 Sep;22(9):2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Gao X. M., Zheng B., Liew F. Y., Brett S., Tite J. Priming of influenza virus-specific cytotoxic T lymphocytes vivo by short synthetic peptides. J Immunol. 1991 Nov 15;147(10):3268–3273. [PubMed] [Google Scholar]
- Hart M. K., Weinhold K. J., Scearce R. M., Washburn E. M., Clark C. A., Palker T. J., Haynes B. F. Priming of anti-human immunodeficiency virus (HIV) CD8+ cytotoxic T cells in vivo by carrier-free HIV synthetic peptides. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9448–9452. doi: 10.1073/pnas.88.21.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Jacobson S., Shida H., McFarlin D. E., Fauci A. S., Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990 Nov 15;348(6298):245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc J. C., Cantor H. T cell-mediated immunity to oncornavirus-induced tumors. II. Ability of different T cell sets to prevent tumor growth in vivo. J Immunol. 1980 Feb;124(2):851–854. [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Karasek M. Isolation and growth of adult human epidermal keratinocytes in cell culture. J Invest Dermatol. 1978 Aug;71(2):157–162. doi: 10.1111/1523-1747.ep12546943. [DOI] [PubMed] [Google Scholar]
- Lombardi S., Garzelli C., La Rosa C., Zaccaro L., Specter S., Malvaldi G., Tozzini F., Esposito F., Bendinelli M. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J Virol. 1993 Aug;67(8):4742–4749. doi: 10.1128/jvi.67.8.4742-4749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Noble G. R., Beare P. A. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M., Reid G., Jarrett O. Immune-stimulating complexes containing Quil A and protein antigen prime class I MHC-restricted T lymphocytes in vivo and are immunogenic by the oral route. Immunology. 1991 Mar;72(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Nixon D. F., Broliden K., Ogg G., Broliden P. A. Cellular and humoral antigenic epitopes in HIV and SIV. Immunology. 1992 Aug;76(4):515–534. [PMC free article] [PubMed] [Google Scholar]
- Nixon D. F., Townsend A. R., Elvin J. G., Rizza C. R., Gallwey J., McMichael A. J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988 Dec 1;336(6198):484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Rowland-Jones S., Nixon D. F., Gotch F. M., Edwards J. P., Ogunlesi A. O., Elvin J. G., Rothbard J. A., Bangham C. R., Rizza C. R. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991 Dec 12;354(6353):453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- Plata F., Dadaglio G., Chenciner N., Hoffenbach A., Wain-Hobson S., Michel F., Langlade-Demoyen P. Cytotoxic T lymphocytes in HIV-induced disease: implications for therapy and vaccination. Immunodefic Rev. 1989;1(3):227–246. [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Rigby M. A., McDonald M., Hosie M. J., Neil J. C., Jarrett O. Immunodiagnosis of feline immunodeficiency virus infection using recombinant viral p17 and p24. AIDS. 1991 Dec;5(12):1477–1483. doi: 10.1097/00002030-199112000-00010. [DOI] [PubMed] [Google Scholar]
- Reusser P., Riddell S. R., Meyers J. D., Greenberg P. D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991 Sep 1;78(5):1373–1380. [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland-Jones S. L., Nixon D. F., Aldhous M. C., Gotch F., Ariyoshi K., Hallam N., Kroll J. S., Froebel K., McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993 Apr 3;341(8849):860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Schulz M., Zinkernagel R. M., Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Näher H., Stroehmann I. Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-specific and HLA-restricted cytotoxic T-cell clones. Nature. 1988 Sep 8;335(6186):178–181. doi: 10.1038/335178a0. [DOI] [PubMed] [Google Scholar]
- Song W., Collisson E. W., Billingsley P. M., Brown W. C. Induction of feline immunodeficiency virus-specific cytolytic T-cell responses from experimentally infected cats. J Virol. 1992 Sep;66(9):5409–5417. doi: 10.1128/jvi.66.9.5409-5417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Webb S. R. Function and specificity of T cell subsets in the mouse. Adv Immunol. 1987;41:39–133. doi: 10.1016/s0065-2776(08)60030-9. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Takeshita T., Morein B., Putney S., Germain R. N., Berzofsky J. A. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
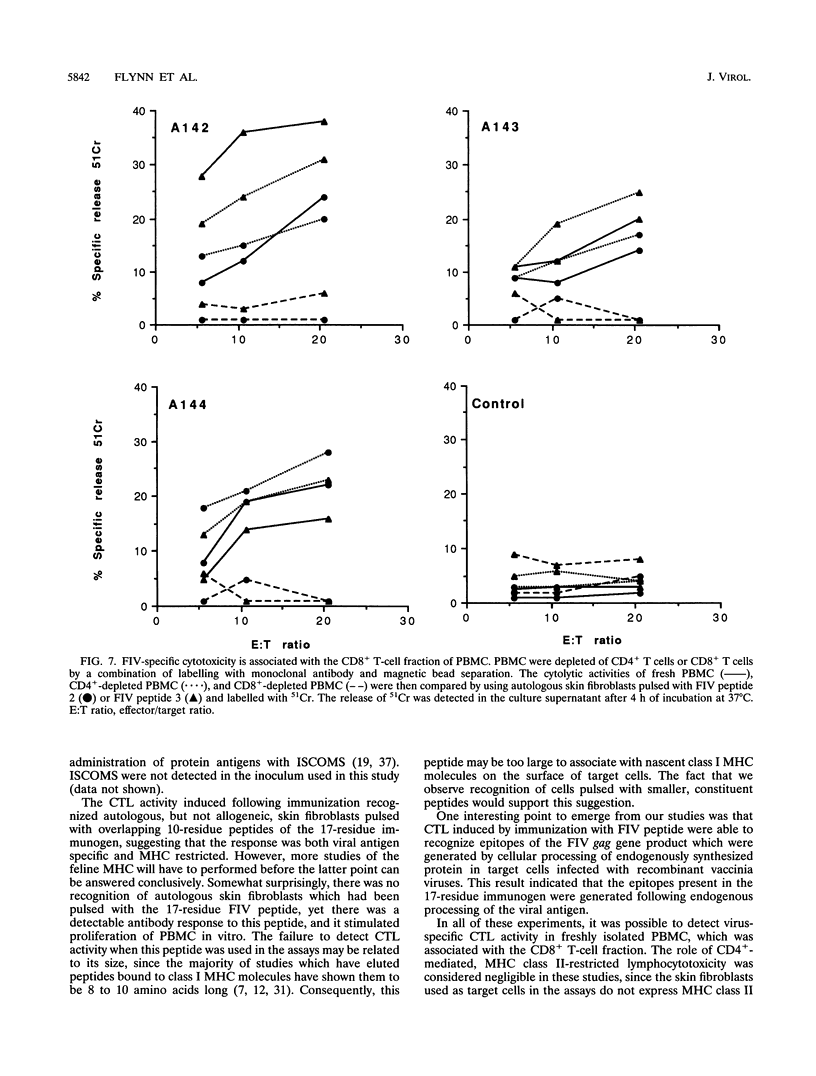
- Willett B. J., Hosie M. J., Callanan J. J., Neil J. C., Jarrett O. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993 Jan;78(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]


