Abstract
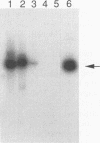
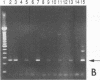
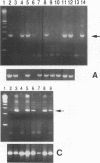
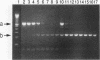
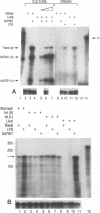
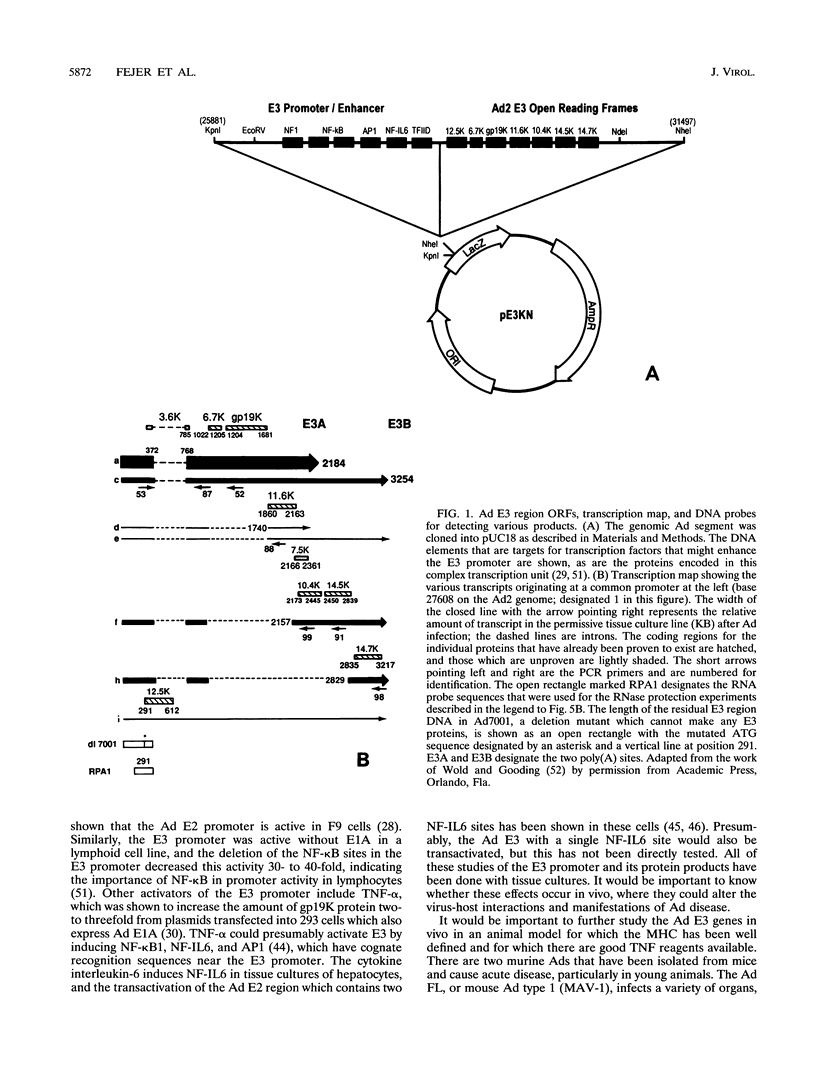
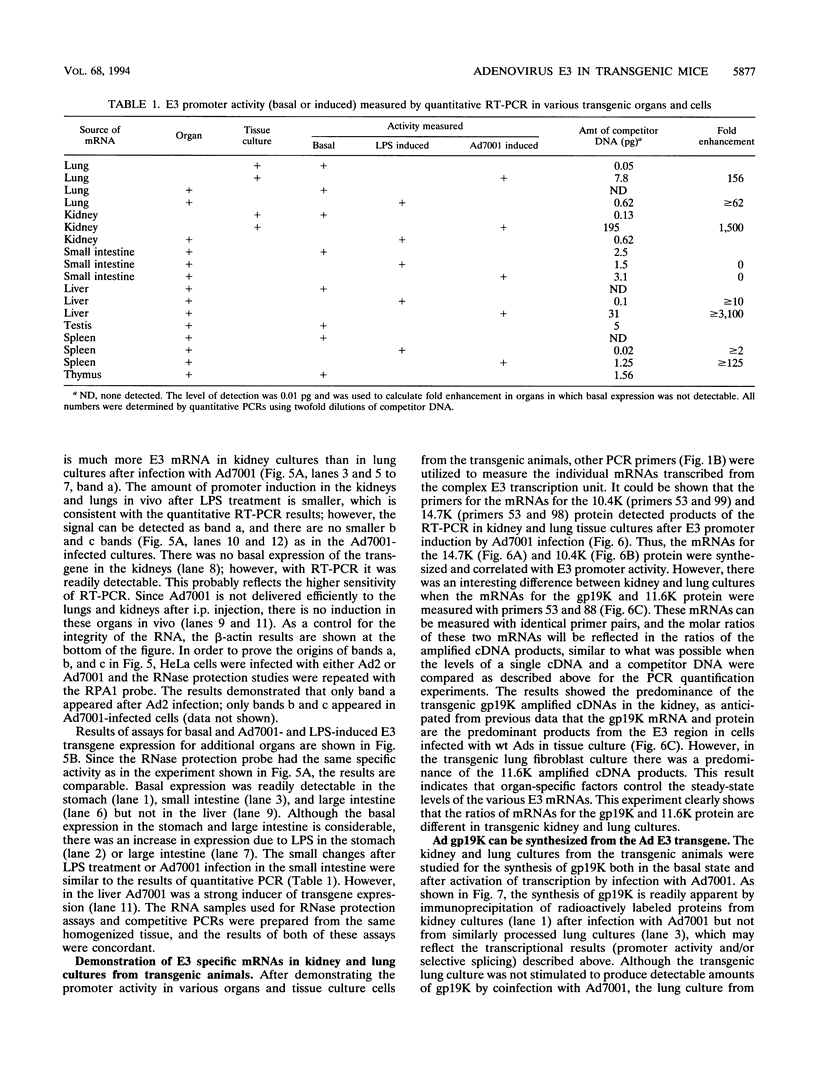
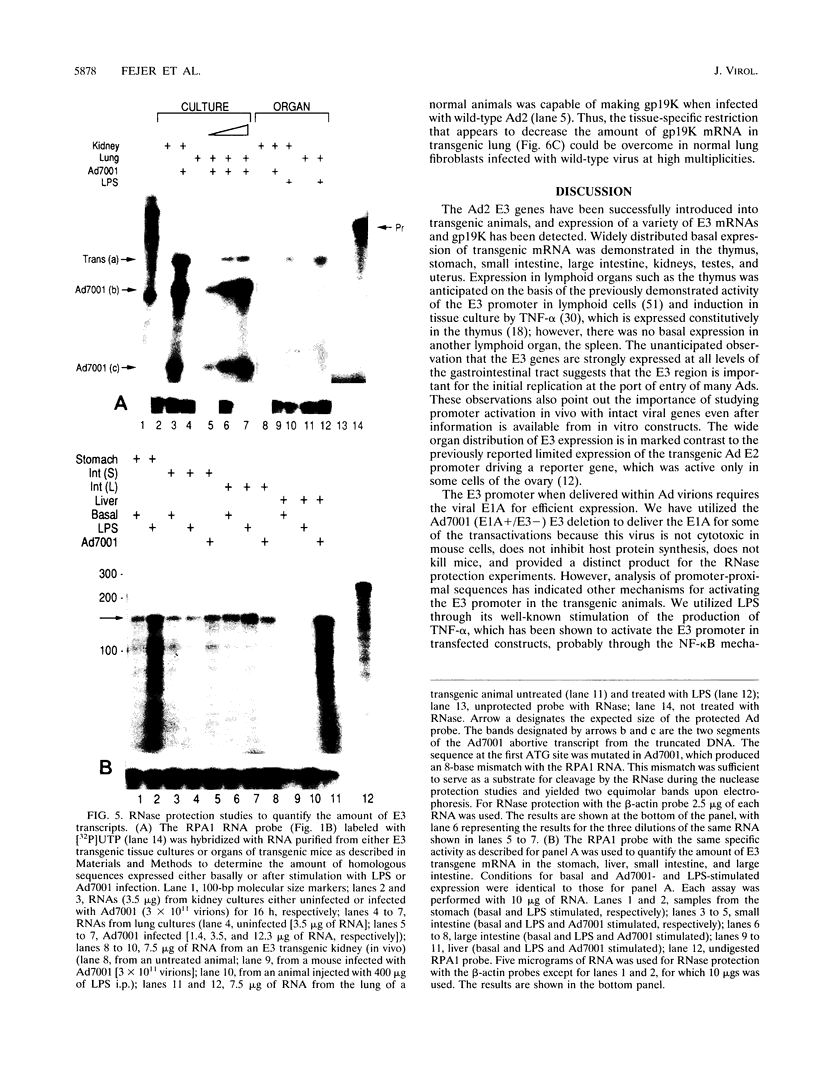
Human adenoviruses (Ad) contain a complex transcription region (E3) which codes for proteins that interact with several arms of the immune system. However, E3 genes are not essential for replication in tissue culture. An E3-encoded 19,000-molecular-weight (19K) glycoprotein (gp19K) binds to the class I major histocompatibility complex (MHC) in the endoplasmic reticulum and prevents MHC transport to the cell surface. Three other E3 proteins are involved in the inhibition of apoptosis by tumor necrosis factor alpha. The entire E3 genomic DNA was utilized to produce transgenic mice to study the effect of the E3 proteins on pathogenesis of various infectious agents and to investigate the in vivo synthesis and processing of the multiple E3 mRNAs and proteins. There was basal expression of the E3 promoter in the thymus, kidneys, uterus, and testes and at all levels of the gastrointestinal tract. In addition, the E3 promoter of the transgene could be activated in some other organs, including the liver, by infection of these animals with an E3-deficient Ad (Ad7001) which contains a functional E1A region. Transactivation in vivo could also be demonstrated by infusion of bacterial lipopolysaccharide. There appeared to be differential ratios of expression between several of the E3 mRNAs in transgenic lung fibroblasts and primary kidney cells cultured from the transgenic animals. This observation suggested that there was differential mRNA splicing that was organ specific. These transgenic animals should provide a useful model for studying the effects of the E3 proteins on the immune system and on diseases affected either by control of MHC or by selected functions of tumor necrosis factor that are inhibitable by Ad E3 proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992 Jun;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Ball A. O., Williams M. E., Spindler K. R. Identification of mouse adenovirus type 1 early region 1: DNA sequence and a conserved transactivating function. J Virol. 1988 Nov;62(11):3947–3957. doi: 10.1128/jvi.62.11.3947-3957.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987 Jul;6(7):2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V. Hepatitis B virus gene expression in transgenic mice. Mol Biol Med. 1989 Apr;6(2):143–149. [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993 Sep;15(3):532-4, 536-7. [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Bhat B., Wold W. S. Mapping the 5' ends, 3' ends, and splice sites of mRNAs from the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):44–54. doi: 10.1016/0042-6822(85)90444-1. [DOI] [PubMed] [Google Scholar]
- Cotten M., Wagner E., Zatloukal K., Birnstiel M. L. Chicken adenovirus (CELO virus) particles augment receptor-mediated DNA delivery to mammalian cells and yield exceptional levels of stable transformants. J Virol. 1993 Jul;67(7):3777–3785. doi: 10.1128/jvi.67.7.3777-3785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley T. P., Miranda M., Jones N. C., DePamphilis M. L. Transactivation of the adenovirus EIIa promoter in the absence of adenovirus E1A protein is restricted to mouse oocytes and preimplantation embryos. Development. 1989 Dec;107(4):945–956. doi: 10.1242/dev.107.4.945. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Horswood R. L., Chanock R. M., Prince G. A. Role of early genes in pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6191–6195. doi: 10.1073/pnas.87.16.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm-Beauchamp U., Horswood R. L., Pernis B., Wold W. S., Chanock R. M., Prince G. A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Moldawer L. L., Sehgal P. B., Redington M., Kilian P. L., Chanock R. M., Prince G. A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroir B. P., Brown T., Beutler B. Constitutive synthesis of tumor necrosis factor in the thymus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4864–4868. doi: 10.1073/pnas.89.11.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Ranheim T. S., Tollefson A. E., Aquino L., Duerksen-Hughes P., Horton T. M., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J Virol. 1991 Aug;65(8):4114–4123. doi: 10.1128/jvi.65.8.4114-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Sofola I. O., Tollefson A. E., Duerksen-Hughes P., Wold W. S. The adenovirus E3-14.7K protein is a general inhibitor of tumor necrosis factor-mediated cytolysis. J Immunol. 1990 Nov 1;145(9):3080–3086. [PubMed] [Google Scholar]
- Hawkins L. K., Wold W. S. A 12,500 MW protein is coded by region E3 of adenovirus. Virology. 1992 Jun;188(2):486–494. doi: 10.1016/0042-6822(92)90502-g. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Intermediates in the synthesis of type 2 adenovirus deoxyribonucleic acid. J Virol. 1971 Nov;8(5):675–683. doi: 10.1128/jvi.8.5.675-683.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kornuc M., Kliewer S., Garcia J., Harrich D., Li C., Gaynor R. Adenovirus early region 3 promoter regulation by E1A/E1B is independent of alterations in DNA binding and gene activation of CREB/ATF and AP1. J Virol. 1990 May;64(5):2004–2013. doi: 10.1128/jvi.64.5.2004-2013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner H., Fritzsche U., Burgert H. G. Tumor necrosis factor alpha stimulates expression of adenovirus early region 3 proteins: implications for viral persistence. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11857–11861. doi: 10.1073/pnas.89.24.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassam N., Feigenbaum L., Vogel J., Jay G. Transgenic approach for the study of pathogenesis induced by human viruses. Mol Biol Med. 1989 Aug;6(4):319–331. [PubMed] [Google Scholar]
- Liu F., Green M. R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990 Jun 29;61(7):1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- Mittal S. K., McDermott M. R., Johnson D. C., Prevec L., Graham F. L. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 1993 Apr;28(1):67–90. doi: 10.1016/0168-1702(93)90090-a. [DOI] [PubMed] [Google Scholar]
- Pirofski L., Horwitz M. S., Scharff M. D., Factor S. M. Murine adenovirus infection of SCID mice induces hepatic lesions that resemble human Reye syndrome. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4358–4362. doi: 10.1073/pnas.88.10.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Ren R., Bouchard M. Poliovirus attenuation and pathogenesis in a transgenic mouse model for poliomyelitis. Dev Biol Stand. 1993;78:109–116. [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Ranheim T. S., Shisler J., Horton T. M., Wold L. J., Gooding L. R., Wold W. S. Characterization of mutants within the gene for the adenovirus E3 14.7-kilodalton protein which prevents cytolysis by tumor necrosis factor. J Virol. 1993 Apr;67(4):2159–2167. doi: 10.1128/jvi.67.4.2159-2167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviprakash K. S., Grunhaus A., el Kholy M. A., Horwitz M. S. The mouse adenovirus type 1 contains an unusual E3 region. J Virol. 1989 Dec;63(12):5455–5458. doi: 10.1128/jvi.63.12.5455-5458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Racaniello V. R. Poliovirus spreads from muscle to the central nervous system by neural pathways. J Infect Dis. 1992 Oct;166(4):747–752. doi: 10.1093/infdis/166.4.747. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Rothe J., Lesslauer W., Lötscher H., Lang Y., Koebel P., Köntgen F., Althage A., Zinkernagel R., Steinmetz M., Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993 Aug 26;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Schütze S., Machleidt T., Krönke M. Mechanisms of tumor necrosis factor action. Semin Oncol. 1992 Apr;19(2 Suppl 4):16–24. [PubMed] [Google Scholar]
- Spergel J. M., Chen-Kiang S. Interleukin 6 enhances a cellular activity that functionally substitutes for E1A protein in transactivation. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6472–6476. doi: 10.1073/pnas.88.15.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel J. M., Hsu W., Akira S., Thimmappaya B., Kishimoto T., Chen-Kiang S. NF-IL6, a member of the C/EBP family, regulates E1A-responsive promoters in the absence of E1A. J Virol. 1992 Feb;66(2):1021–1030. doi: 10.1128/jvi.66.2.1021-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson A. E., Stewart A. R., Yei S. P., Saha S. K., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus form a complex and function together to down-regulate the epidermal growth factor receptor. J Virol. 1991 Jun;65(6):3095–3105. doi: 10.1128/jvi.65.6.3095-3105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufariello J., Cho S., Horwitz M. S. The adenovirus E3 14.7-kilodalton protein which inhibits cytolysis by tumor necrosis factor increases the virulence of vaccinia virus in a murine pneumonia model. J Virol. 1994 Jan;68(1):453–462. doi: 10.1128/jvi.68.1.453-462.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Napolitano L. A., Ngo L., Jay G. Liver cancer in transgenic mice carrying the human immunodeficiency virus tat gene. Cancer Res. 1991 Dec 15;51(24):6686–6690. [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Williams J. L., Garcia J., Harrich D., Pearson L., Wu F., Gaynor R. Lymphoid specific gene expression of the adenovirus early region 3 promoter is mediated by NF-kappa B binding motifs. EMBO J. 1990 Dec;9(13):4435–4442. doi: 10.1002/j.1460-2075.1990.tb07894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991 Sep;184(1):1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- Zabner J., Couture L. A., Gregory R. J., Graham S. M., Smith A. E., Welsh M. J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993 Oct 22;75(2):207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]