Abstract
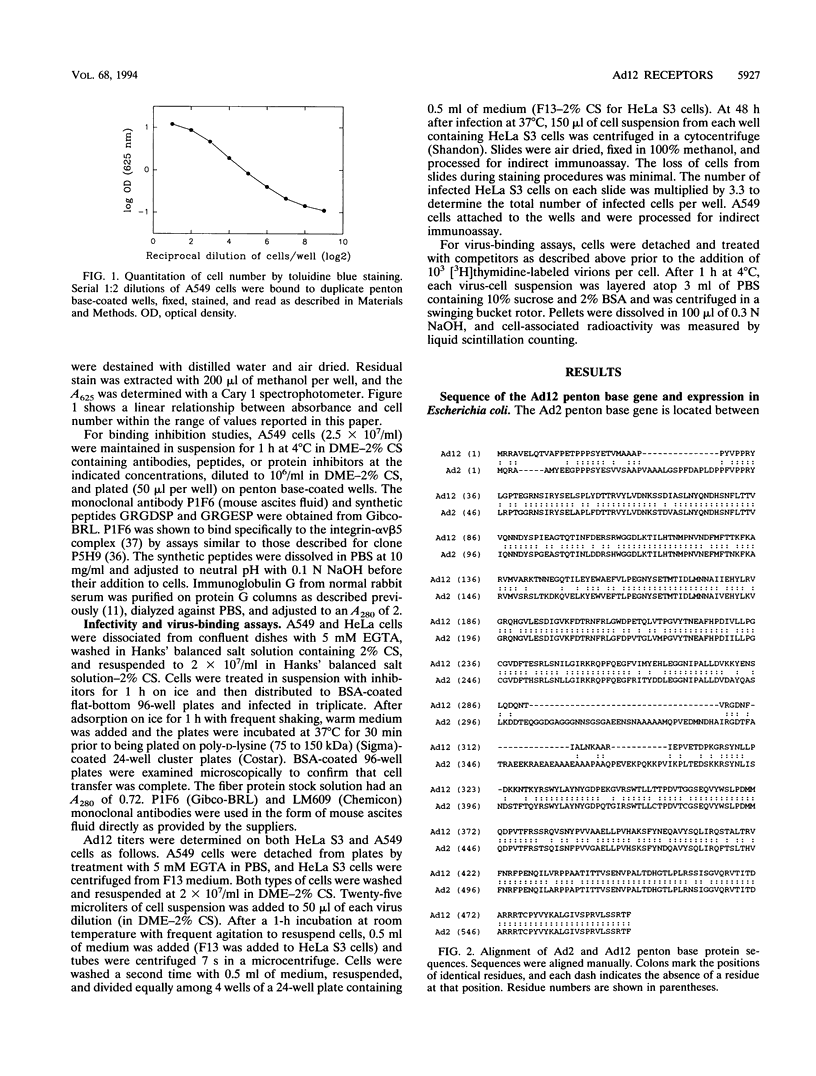
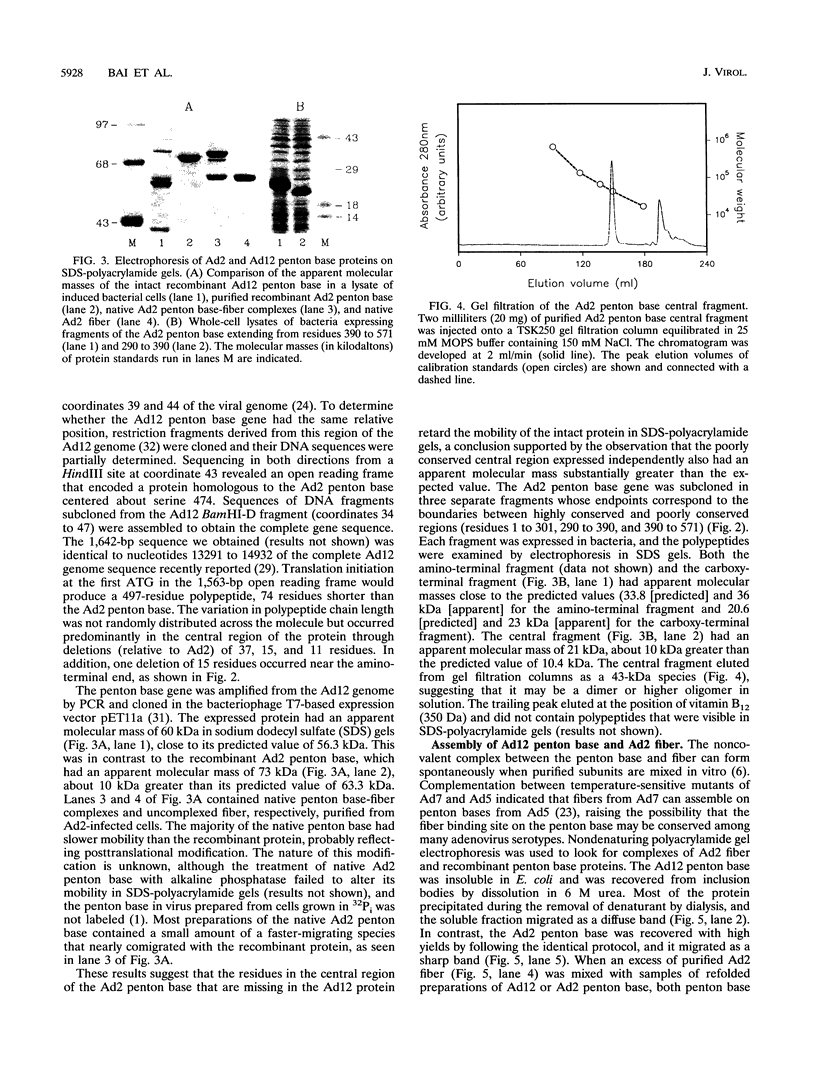
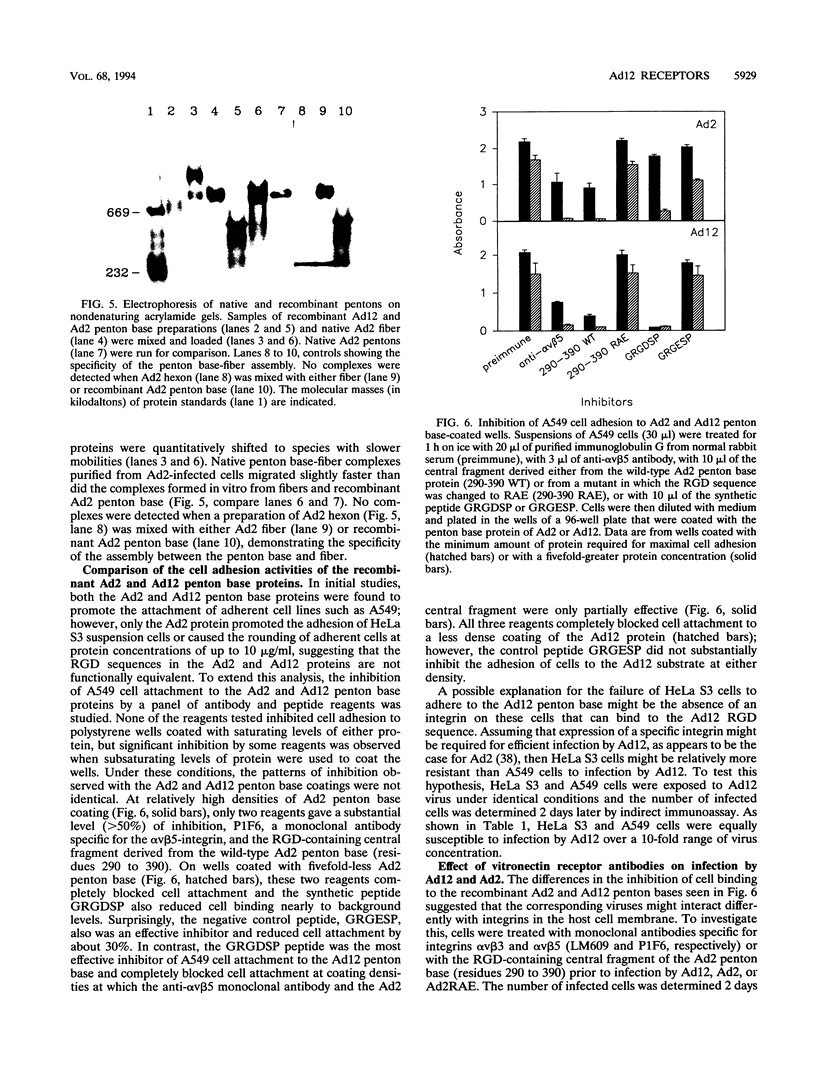
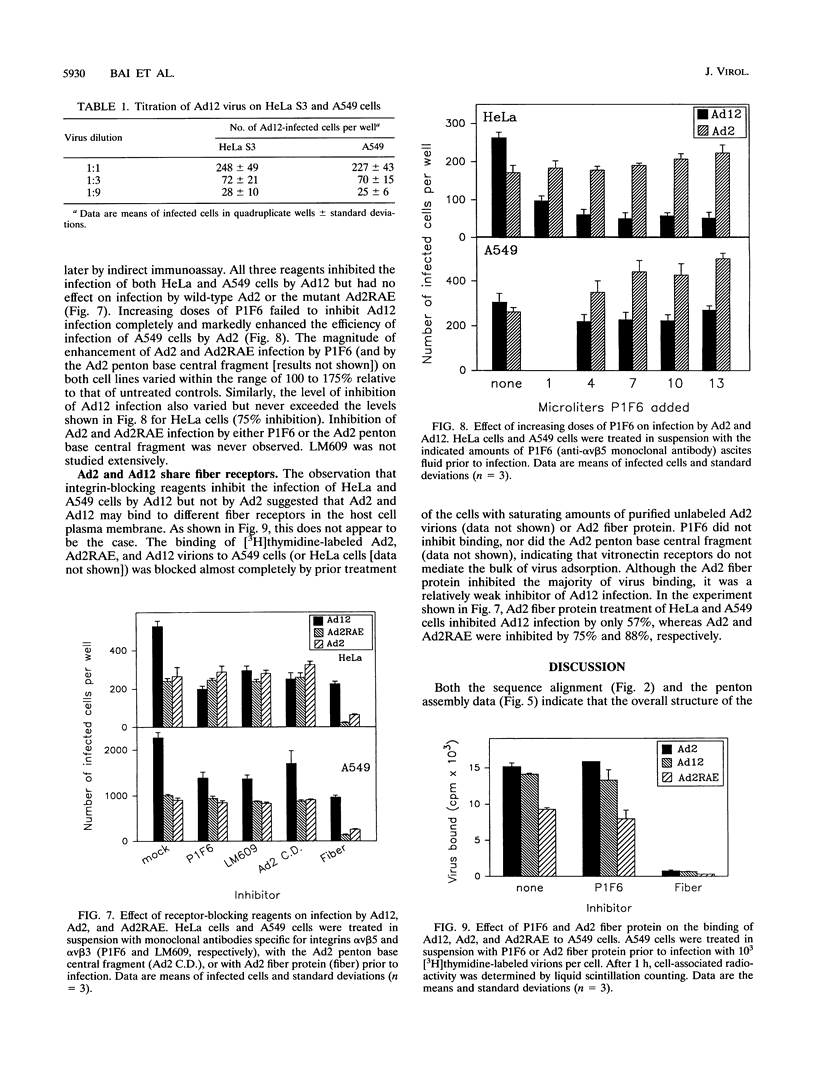
The penton base gene from adenovirus type 12 (Ad12) was sequenced and encodes a 497-residue polypeptide, 74 residues shorter than the penton base from Ad2. The Ad2 and Ad12 proteins are highly conserved at the amino- and carboxy-terminal ends but diverge radically in the central region, where 63 residues are missing from the Ad12 sequence. Conserved within this variable region is the sequence Arg-Gly-Asp (RGD), which, in the Ad2 penton base, binds to integrins in the target cell membrane, enhancing the rate or the efficiency of infection. The Ad12 penton base was expressed in Escherichia coli, and the purified refolded protein assembled in vitro with Ad2 fibers. In contrast to the Ad2 penton base, the Ad12 protein failed to cause the rounding of adherent cells or to promote attachment of HeLa S3 suspension cells; however, A549 cells did attach to surfaces coated with either protein and pretreatment of the cells with an integrin alpha v beta 5 monoclonal antibody reduced attachment to background levels. Treatment of HeLa and A549 cells with integrin alpha v beta 3 or alpha v beta 5 monoclonal antibodies or with an RGD-containing fragment of the Ad2 penton base protein inhibited infection by Ad12 but had no effect on and in some cases enhanced infection by Ad2. Purified Ad2 fiber protein reduced the binding of radiolabeled Ad2 and Ad12 virions to HeLa and A549 cells nearly to background levels, but the concentrations of fiber that strongly inhibited infection by Ad2 only weakly inhibited Ad12 infection. These data suggest that alpha v-containing integrins alone may be sufficient to support infection by Ad12 and that this pathway is not efficiently used by Ad2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aumailley M., Gurrath M., Müller G., Calvete J., Timpl R., Kessler H. Arg-Gly-Asp constrained within cyclic pentapeptides. Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991 Oct 7;291(1):50–54. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- Bai M., Harfe B., Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993 Sep;67(9):5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin M. T., Boulanger P. Involvement of cellular adhesion sequences in the attachment of adenovirus to the HeLa cell surface. J Gen Virol. 1993 Aug;74(Pt 8):1485–1497. doi: 10.1099/0022-1317-74-8-1485. [DOI] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., Boulanger P. Assembly of adenovirus penton base and fiber. Virology. 1982 Jan 30;116(2):589–604. doi: 10.1016/0042-6822(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Puvion F. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur J Biochem. 1973 Nov 1;39(1):37–42. doi: 10.1111/j.1432-1033.1973.tb03100.x. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Telford E. A., Watson M. S., McBride K., Mautner V. The DNA sequence of adenovirus type 40. J Mol Biol. 1993 Dec 20;234(4):1308–1316. doi: 10.1006/jmbi.1993.1687. [DOI] [PubMed] [Google Scholar]
- Defer C., Belin M. T., Caillet-Boudin M. L., Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990 Aug;64(8):3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERETT S. F., GINSBERG H. S. A toxin-like material separable from type 5 adenovirus particles. Virology. 1958 Dec;6(3):770–771. doi: 10.1016/0042-6822(58)90123-5. [DOI] [PubMed] [Google Scholar]
- Hennache B., Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977 Aug 15;166(2):237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- Louis N., Fender P., Barge A., Kitts P., Chroboczek J. Cell-binding domain of adenovirus serotype 2 fiber. J Virol. 1994 Jun;68(6):4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREIRA H. G. A protein factor responsible for the early cytopathic effect of adenoviruses. Virology. 1958 Dec;6(3):601–611. doi: 10.1016/0042-6822(58)90109-0. [DOI] [PubMed] [Google Scholar]
- PEREIRA H. G., DE FIGUEIREDO M. V. Mechanism of hemagglutination by adenovirus types 1,2,4,5 and 6. Virology. 1962 Sep;18:1–8. doi: 10.1016/0042-6822(62)90171-x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praszkier J., Ginsberg H. S. Isolation and characterization of temperature-sensitive mutants of adenovirus type 7. J Virol. 1987 Oct;61(10):3089–3095. doi: 10.1128/jvi.61.10.3089-3095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ROIZMAN B., LEVY H. B. Characterization of a factor formed in the course of adenovirus infection of tissue cultures causing detachment of cells from glass. J Exp Med. 1958 Nov 1;108(5):713–729. doi: 10.1084/jem.108.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sheppard M., Trist H. Characterization of the avian adenovirus penton base. Virology. 1992 Jun;188(2):881–886. doi: 10.1016/0042-6822(92)90546-2. [DOI] [PubMed] [Google Scholar]
- Silver L., Anderson C. W. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988 Aug;165(2):377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- Sprengel J., Schmitz B., Heuss-Neitzel D., Zock C., Doerfler W. Nucleotide sequence of human adenovirus type 12 DNA: comparative functional analysis. J Virol. 1994 Jan;68(1):379–389. doi: 10.1128/jvi.68.1.379-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. L., Fuller S. D., Burnett R. M. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993 Jul;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Svensson U., Persson R., Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., Norrby E. Immunological and other biological characteristics of pentons of human adenoviruses. J Virol. 1969 Nov;4(5):671–680. doi: 10.1128/jvi.4.5.671-680.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinacker A., Chen A., Agrez M., Cone R. I., Nishimura S., Wayner E., Pytela R., Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994 Mar 4;269(9):6940–6948. [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Oostrum J., Burnett R. M. Molecular composition of the adenovirus type 2 virion. J Virol. 1985 Nov;56(2):439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]