Abstract
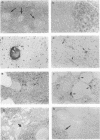
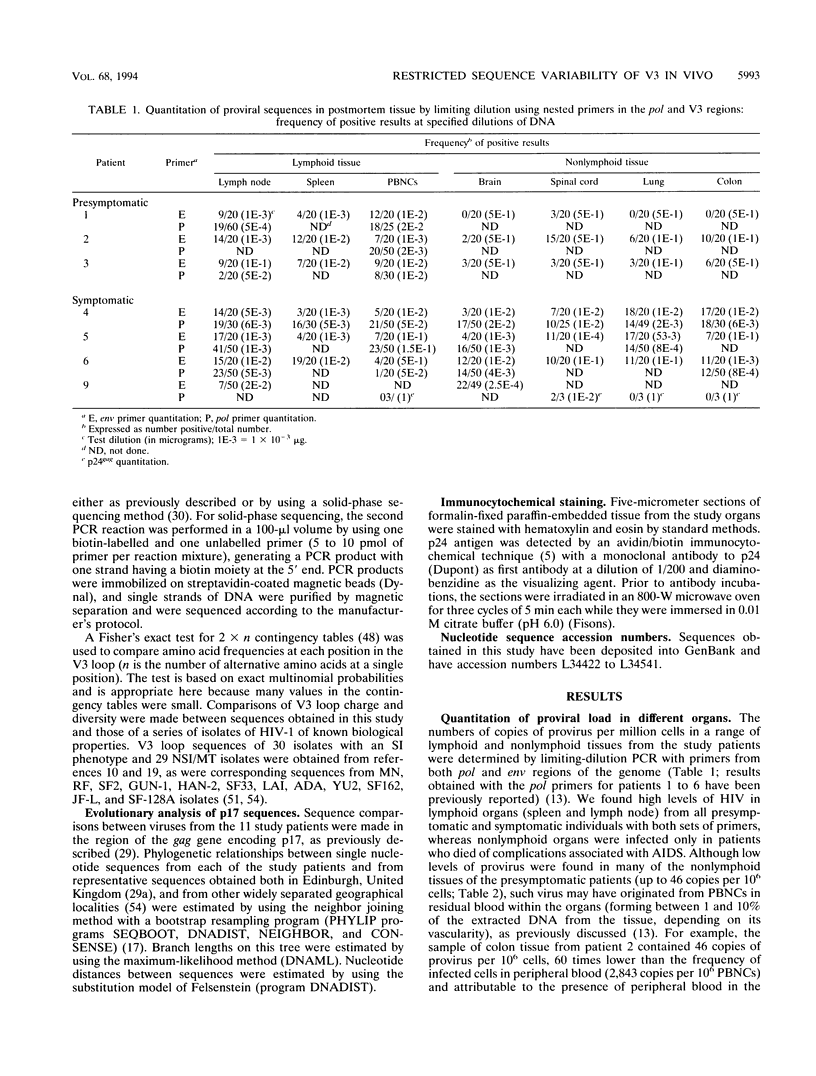
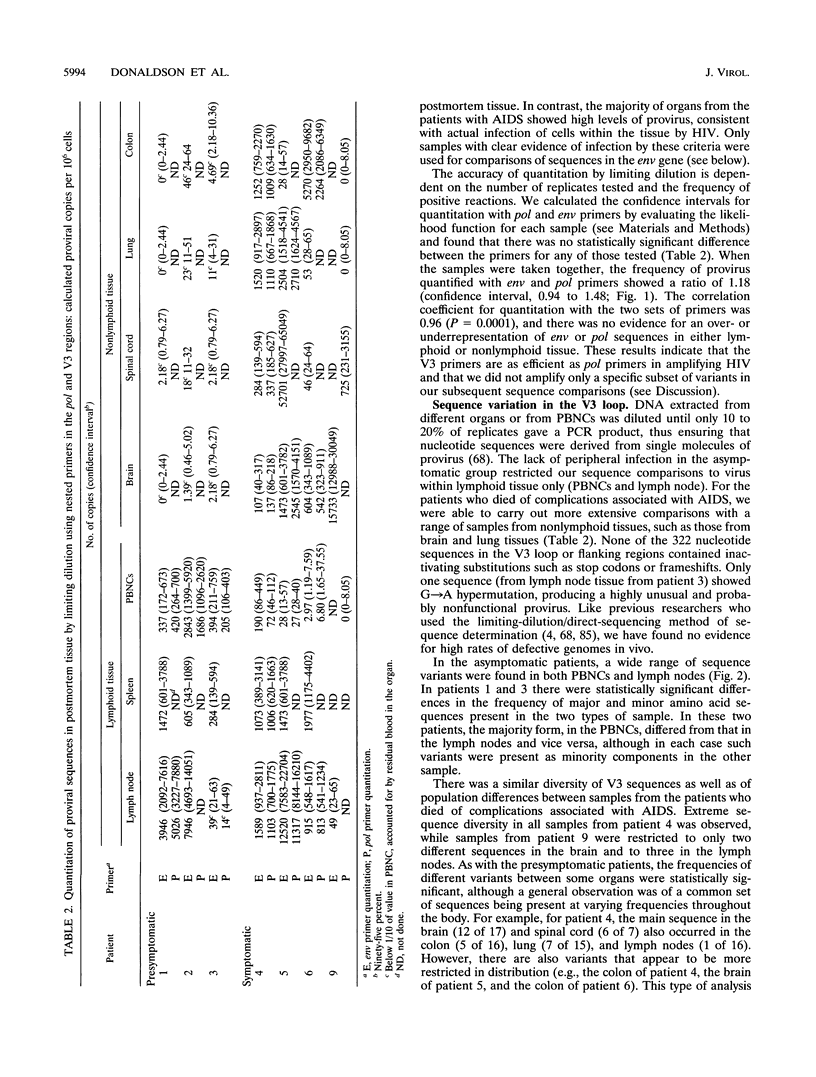
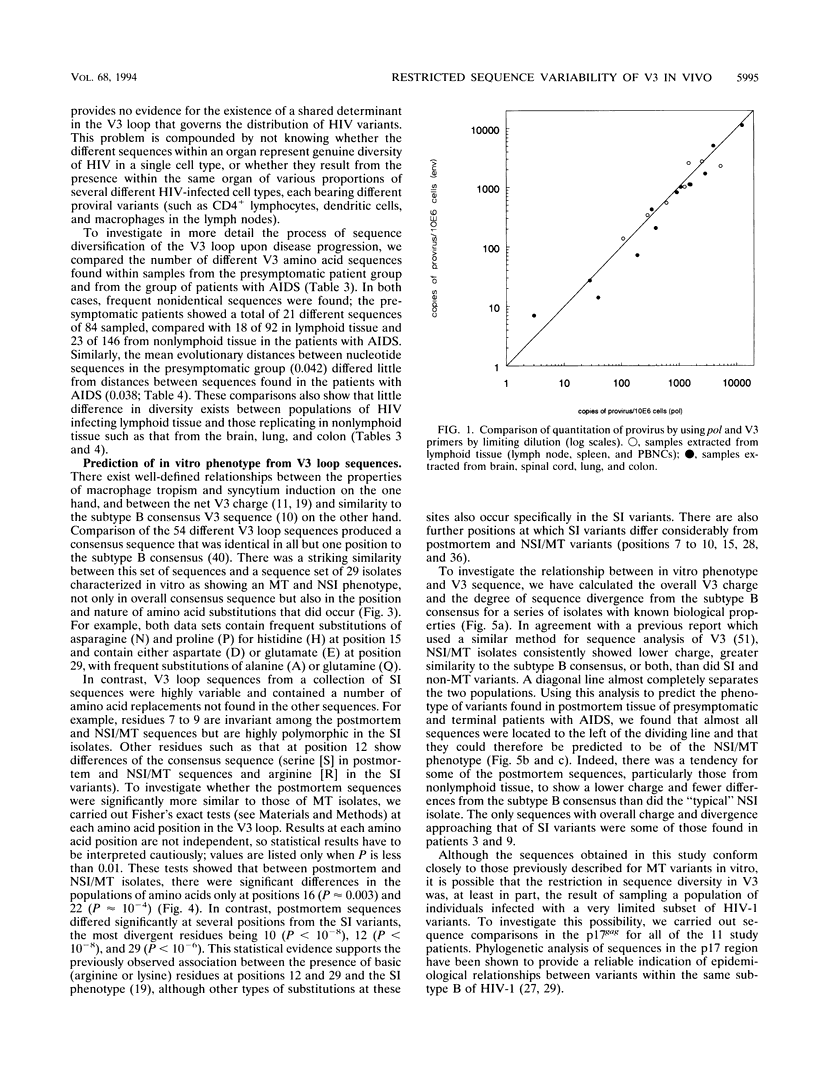
The distribution, cell tropism, and cytopathology in vivo of human immunodeficiency virus (HIV) was investigated in postmortem tissue samples from a series of HIV-infected individuals who died either of complications associated with AIDS or for unrelated reasons while they were asymptomatic. Proviral sequences were detected at a high copy number in lymphoid tissue of both presymptomatic patients and patients with AIDS, whereas significant infection of nonlymphoid tissue such as that from brains, spinal cords, and lungs were confined to those with AIDS. V3 loop sequences from both groups showed highly restricted sequence variability and a low overall positive charge of the encoded amino acid sequence compared with those of standard laboratory isolates of HIV type 1 (HIV-1). The low charge and the restriction in sequence variability were comparable to those observed with isolates showing a non-syncytium-inducing (NSI) and macrophage-tropic phenotype in vitro. All patients were either exclusively infected (six of seven cases) or predominantly infected (one case) with variants with a predicted NSI/macrophage-tropic phenotype, irrespective of the degree of disease progression. p24 antigen was detected by immunocytochemical staining of paraffin-fixed sections in the germinal centers within lymphoid tissue, although little or no antigen was found in areas of lymph node or spleen containing T lymphocytes from either presymptomatic patients or patients with AIDS. The predominant p24 antigen-expressing cells in the lungs and brains of the patients with AIDS were macrophages and microglia (in brains), frequently forming multinucleated giant cells (syncytia) even though the V3 loop sequences of these variants resembled those of NSI isolates in vitro. These studies indicate that lack of syncytium-forming ability in established T-cell lines does not necessarily predict syncytium-forming ability in primary target cells in vivo. Furthermore, variants of HIV with V3 sequences characteristic of NSI/macrophage-tropic isolates form the predominant population in a range of lymphoid and nonlymphoid tissues in vivo, even in patients with AIDS.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond N., Jenkins A., Heath A. B., Kitchin P. Sequence variation in the env gene of simian immunodeficiency virus recovered from immunized macaques is predominantly in the V1 region. J Gen Virol. 1993 May;74(Pt 5):865–871. doi: 10.1099/0022-1317-74-5-865. [DOI] [PubMed] [Google Scholar]
- Andeweg A. C., Leeflang P., Osterhaus A. D., Bosch M. L. Both the V2 and V3 regions of the human immunodeficiency virus type 1 surface glycoprotein functionally interact with other envelope regions in syncytium formation. J Virol. 1993 Jun;67(6):3232–3239. doi: 10.1128/jvi.67.6.3232-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Balfe P., Simmonds P., Ludlam C. A., Bishop J. O., Brown A. J. Concurrent evolution of human immunodeficiency virus type 1 in patients infected from the same source: rate of sequence change and low frequency of inactivating mutations. J Virol. 1990 Dec;64(12):6221–6233. doi: 10.1128/jvi.64.12.6221-6233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. E., Busuttil A., Ironside J. W., Rebus S., Donaldson Y. K., Simmonds P., Peutherer J. F. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J Infect Dis. 1993 Oct;168(4):818–824. doi: 10.1093/infdis/168.4.818. [DOI] [PubMed] [Google Scholar]
- Boyd M. T., Simpson G. R., Cann A. J., Johnson M. A., Weiss R. A. A single amino acid substitution in the V1 loop of human immunodeficiency virus type 1 gp120 alters cellular tropism. J Virol. 1993 Jun;67(6):3649–3652. doi: 10.1128/jvi.67.6.3649-3652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. P., Desrosiers R. C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991 Apr;65(4):1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Weiss C., Seto D., Levy J. A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong J. J., De Ronde A., Keulen W., Tersmette M., Goudsmit J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J Virol. 1992 Nov;66(11):6777–6780. doi: 10.1128/jvi.66.11.6777-6780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson Y. K., Bell J. E., Ironside J. W., Brettle R. P., Robertson J. R., Busuttil A., Simmonds P. Redistribution of HIV outside the lymphoid system with onset of AIDS. Lancet. 1994 Feb 12;343(8894):383–385. doi: 10.1016/s0140-6736(94)91222-x. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Beneke J., Till M., Wolinsky S., Ribas J. L., Burke A., Haase A. T. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Kuiken C., Blumberg B. M., Hartman S., Sharer L. R., Clement M., Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991 Feb;180(2):583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R. A., Groenink M., Kootstra N. A., Tersmette M., Huisman H. G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992 May;66(5):3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. S., Sun C. R., Gordon W. L., Liou R. S., Chang T. W., Sun W. N., Daar E. S., Ho D. D. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J Virol. 1992 Feb;66(2):848–856. doi: 10.1128/jvi.66.2.848-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Cara A., Gallo R. C., Lori F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Husayni H., Turpin J. A., Skillman D., Kalter D. C., Orenstein J. M., Hoover D. L., Meltzer M. S. Macrophage-HIV interaction: viral isolation and target cell tropism. AIDS. 1990 Mar;4(3):221–228. [PubMed] [Google Scholar]
- Groenink M., Fouchier R. A., Broersen S., Baker C. H., Koot M., van't Wout A. B., Huisman H. G., Miedema F., Tersmette M., Schuitemaker H. Relation of phenotype evolution of HIV-1 to envelope V2 configuration. Science. 1993 Jun 4;260(5113):1513–1516. doi: 10.1126/science.8502996. [DOI] [PubMed] [Google Scholar]
- Haggerty S., Stevenson M. Predominance of distinct viral genotypes in brain and lymph node compartments of HIV-1-infected individuals. Viral Immunol. 1991 Summer;4(2):123–131. doi: 10.1089/vim.1991.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigwood N. L., Shuster J. R., Moore G. K., Lee H., Skiles P. V., Higgins K. W., Barr P. J., George-Nascimento C., Steimer K. S. Importance of hypervariable regions of HIV-1 gp120 in the generation of virus neutralizing antibodies. AIDS Res Hum Retroviruses. 1990 Jul;6(7):855–869. doi: 10.1089/aid.1990.6.855. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Brown A. J., Simmonds P. Sequence data as evidence. Nature. 1993 Aug 26;364(6440):766–766. doi: 10.1038/364766b0. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Zhang L. Q., Simmonds P., Ludlam C. A., Brown A. J. Convergent and divergent sequence evolution in the surface envelope glycoprotein of human immunodeficiency virus type 1 within a single infected patient. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4835–4839. doi: 10.1073/pnas.89.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. C., Zhang L. Q., Simmonds P., Rogers A. S., Brown A. J. Molecular investigation of human immunodeficiency virus (HIV) infection in a patient of an HIV-infected surgeon. J Infect Dis. 1993 Jun;167(6):1411–1414. doi: 10.1093/infdis/167.6.1411. [DOI] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Keys B., Karis J., Fadeel B., Valentin A., Norkrans G., Hagberg L., Chiodi F. V3 sequences of paired HIV-1 isolates from blood and cerebrospinal fluid cluster according to host and show variation related to the clinical stage of disease. Virology. 1993 Oct;196(2):475–483. doi: 10.1006/viro.1993.1503. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Koot M., Keet I. P., Vos A. H., de Goede R. E., Roos M. T., Coutinho R. A., Miedema F., Schellekens P. T., Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993 May 1;118(9):681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kuiken C. L., Zwart G., Baan E., Coutinho R. A., van den Hoek J. A., Goudsmit J. Increasing antigenic and genetic diversity of the V3 variable domain of the human immunodeficiency virus envelope protein in the course of the AIDS epidemic. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9061–9065. doi: 10.1073/pnas.90.19.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken C. L., de Jong J. J., Baan E., Keulen W., Tersmette M., Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992 Jul;66(7):4622–4627. doi: 10.1128/jvi.66.7.4622-4627.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi K., Conway B., Cunningham S., Berson A., Evans C., Iversen A. K., Colvin D., Gallo M. V., Coutre S., Shpaer E. G. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J Virol. 1992 Feb;66(2):875–885. doi: 10.1128/jvi.66.2.875-885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kappes J. C., Conway J. A., Price R. W., Shaw G. M., Hahn B. H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991 Aug;65(8):3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Q., Wood C., Levy J. A., Cheng-Mayer C. The viral envelope gene is involved in macrophage tropism of a human immunodeficiency virus type 1 strain isolated from brain tissue. J Virol. 1990 Dec;64(12):6148–6153. doi: 10.1128/jvi.64.12.6148-6153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney D. J., Fisher A. G., Putney S. D., Rusche J. R., Redfield R. R., Burke D. S., Gallo R. C., Wong-Staal F. Type-restricted neutralization of molecular clones of human immunodeficiency virus. Science. 1988 Jul 15;241(4863):357–359. doi: 10.1126/science.3388046. [DOI] [PubMed] [Google Scholar]
- Lori F., di Marzo Veronese F., de Vico A. L., Lusso P., Reitz M. S., Jr, Gallo R. C. Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol. 1992 Aug;66(8):5067–5074. doi: 10.1128/jvi.66.8.5067-5074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie J., McCutchan F. E., Peeters M., Brennan T. P., Sanders-Buell E., Eddy G. A., van der Groen G., Fransen K., Gershy-Damet G. M., Deleys R. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993 Jun;7(6):769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Lau R., Patterson S., Pinching A. J., Knight S. C. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990 Sep;71(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- Meloen R. H., Liskamp R. M., Goudsmit J. Specificity and function of the individual amino acids of an important determinant of human immunodeficiency virus type 1 that induces neutralizing activity. J Gen Virol. 1989 Jun;70(Pt 6):1505–1512. doi: 10.1099/0022-1317-70-6-1505. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Milich L., Margolin B., Swanstrom R. V3 loop of the human immunodeficiency virus type 1 Env protein: interpreting sequence variability. J Virol. 1993 Sep;67(9):5623–5634. doi: 10.1128/jvi.67.9.5623-5634.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., MacIsaac P. D., Torbett B. E., Levy J. A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993 Apr 30;260(5108):689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Ou C. Y., Ciesielski C. A., Myers G., Bandea C. I., Luo C. C., Korber B. T., Mullins J. I., Schochetman G., Berkelman R. L., Economou A. N. Molecular epidemiology of HIV transmission in a dental practice. Science. 1992 May 22;256(5060):1165–1171. doi: 10.1126/science.256.5060.1165. [DOI] [PubMed] [Google Scholar]
- Pang S., Vinters H. V., Akashi T., O'Brien W. A., Chen I. S. HIV-1 env sequence variation in brain tissue of patients with AIDS-related neurologic disease. J Acquir Immune Defic Syndr. 1991;4(11):1082–1092. [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Roos M. T., Lange J. M., de Goede R. E., Coutinho R. A., Schellekens P. T., Miedema F., Tersmette M. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis. 1992 Mar;165(3):427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti G., Leitner T., Halapi E., Wahlberg J., Marchisio P., Clerici-Schoeller M. A., Wigzell H., Fenyö E. M., Albert J., Uhlén M. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with the RNA and DNA sequences of the virus populations of their mothers. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1721–1725. doi: 10.1073/pnas.90.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker H., Koot M., Kootstra N. A., Dercksen M. W., de Goede R. E., van Steenwijk R. P., Lange J. M., Schattenkerk J. K., Miedema F., Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992 Mar;66(3):1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker H., Kootstra N. A., de Goede R. E., de Wolf F., Miedema F., Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991 Jan;65(1):356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker H., Meyaard L., Kootstra N. A., Dubbes R., Otto S. A., Tersmette M., Heeney J. L., Miedema F. Lack of T cell dysfunction and programmed cell death in human immunodeficiency virus type 1-infected chimpanzees correlates with absence of monocytotropic variants. J Infect Dis. 1993 Nov;168(5):1140–1147. doi: 10.1093/infdis/168.5.1140. [DOI] [PubMed] [Google Scholar]
- Sharma D. P., Zink M. C., Anderson M., Adams R., Clements J. E., Joag S. V., Narayan O. Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J Virol. 1992 Jun;66(6):3550–3556. doi: 10.1128/jvi.66.6.3550-3556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Ludlam C. A., Bishop J. O., Brown A. J. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J Virol. 1990 Dec;64(12):5840–5850. doi: 10.1128/jvi.64.12.5840-5850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., McOmish F., Balfe P., Ludlam C. A., Brown A. J. Discontinuous sequence change of human immunodeficiency virus (HIV) type 1 env sequences in plasma viral and lymphocyte-associated proviral populations in vivo: implications for models of HIV pathogenesis. J Virol. 1991 Nov;65(11):6266–6276. doi: 10.1128/jvi.65.11.6266-6276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Steuler H., Storch-Hagenlocher B., Wildemann B. Distinct populations of human immunodeficiency virus type 1 in blood and cerebrospinal fluid. AIDS Res Hum Retroviruses. 1992 Jan;8(1):53–59. doi: 10.1089/aid.1992.8.53. [DOI] [PubMed] [Google Scholar]
- Sullivan N., Thali M., Furman C., Ho D. D., Sodroski J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J Virol. 1993 Jun;67(6):3674–3679. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Palomino S., Rojas J. M., Martínez M. A., Fenyö E. M., Nájera R., Domingo E., López-Galíndez C. Dilute passage promotes expression of genetic and phenotypic variants of human immunodeficiency virus type 1 in cell culture. J Virol. 1993 May;67(5):2938–2943. doi: 10.1128/jvi.67.5.2938-2943.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Akutsu M., Murayama K., Shimizu N., Hoshino H. Host range mutant of human immunodeficiency virus type 1: modification of cell tropism by a single point mutation at the neutralization epitope in the env gene. J Virol. 1991 Apr;65(4):1710–1718. doi: 10.1128/jvi.65.4.1710-1718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., Lange J. M., de Goede R. E., de Wolf F., Eeftink-Schattenkerk J. K., Schellekens P. T., Coutinho R. A., Huisman J. G., Goudsmit J., Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989 May 6;1(8645):983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992 Aug;66(8):4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins B. A., Dorn H. H., Kelly W. B., Armstrong R. C., Potts B. J., Michaels F., Kufta C. V., Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990 Aug 3;249(4968):549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Rutledge R. A., Dias S., Folks T., Theodore T., Buckler C. E., Martin M. A. Identification of conserved and divergent domains within the envelope gene of the acquired immunodeficiency syndrome retrovirus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5038–5042. doi: 10.1073/pnas.83.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992 Jul;189(1):103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- Wolfs T. F., de Jong J. J., Van den Berg H., Tijnagel J. M., Krone W. J., Goudsmit J. Evolution of sequences encoding the principal neutralization epitope of human immunodeficiency virus 1 is host dependent, rapid, and continuous. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9938–9942. doi: 10.1073/pnas.87.24.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky S. M., Wike C. M., Korber B. T., Hutto C., Parks W. P., Rosenblum L. L., Kunstman K. J., Furtado M. R., Muñoz J. L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992 Feb 28;255(5048):1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang Y., Spicer T. P., Abbott L. Z., Abbott M., Poiesz B. J. Reverse transcription takes place within extracellular HIV-1 virions: potential biological significance. AIDS Res Hum Retroviruses. 1993 Dec;9(12):1287–1296. doi: 10.1089/aid.1993.9.1287. [DOI] [PubMed] [Google Scholar]
- Zhang L. Q., MacKenzie P., Cleland A., Holmes E. C., Brown A. J., Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993 Jun;67(6):3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. M., Dawson S. C., Landsman D., Lane H. C., Salzman N. P. Persistence of four related human immunodeficiency virus subtypes during the course of zidovudine therapy: relationship between virion RNA and proviral DNA. J Virol. 1994 Jan;68(1):425–432. doi: 10.1128/jvi.68.1.425-432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Mo H., Wang N., Nam D. S., Cao Y., Koup R. A., Ho D. D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993 Aug 27;261(5125):1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- de Jong J. J., Goudsmit J., Keulen W., Klaver B., Krone W., Tersmette M., de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992 Feb;66(2):757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]