Abstract
Transplantations of fully allogeneic, autoimmune-resistant T-cell-depleted marrow (TCDM) plus syngeneic, autoimmune-prone TCDM into lethally irradiated BXSB mice were carried out to investigate the ability of the mixed bone marrow transplantation (BMT) to prevent development of autoimmune disease and, at the same time, to reconstitute fully the immunity functions of heavily irradiated BXSB recipients. Male BXSB mice were engrafted with mixed TCDM from both allogeneic, autoimmune-resistant BALB/c mice and syngeneic, autoimmune-prone BXSB mice. BMT with mixed TCDM from both resistant and susceptible strains of mice (mixed BMT) prolonged the median life span and inhibited development of glomerulonephritis in BXSB mice. BMT with mixed TCDM also prevented the formation of anti-DNA antibodies that is typically observed in male mice of this strain. Moreover, mixed BMT reconstituted primary antibody production in BXSB recipients, so that no annoying immunodeficiencies that are regularly observed in fully allogeneic chimeras were present in the recipient of the mixed TCDM. These findings indicate that transplanting allogeneic, autoimmune-resistant TCDM plus syngeneic, autoimmune-prone TCDM into lethally irradiated BXSB mice prevents development of autoimmune disease in this strain of mice. In addition, this dual BMT reconstitutes the immunity functions and avoids the immunodeficiencies that occur regularly in fully allogeneic chimeras after total-body irradiation.
The etiologic and pathogenetic bases of many autoimmune diseases in relatively short-lived inbred strains of mice ultimately reside in the primitive, self-renewing hematopoietic stem cell population. The effects of bone marrow transplantation (BMT) and other forms of cellular engineering as treatment and/or prevention of these autoimmune diseases in mice have been investigated extensively (1–8). Cellular engineering by transplantation strategies, which replace the primitive self-renewing hematopoietic stem cells of the recipient with those of the donor, can be used to treat or prevent many autoimmune diseases in mice. It has been established that fully allogeneic BMT, after purging the marrow of destructive T cells, can prolong the span of life, inhibit the production of serum autoantibodies, and treat or prevent the development of the autoimmune-associated histopathological lesions in autoimmune-prone strains of mice (1–5). However, the fully allogeneic chimeras with donor and recipient fully mismatched at the major histocompatibility complex (MHC) experience immunodeficiencies after total-body irradiation (TBI) followed by BMT. Although these fully allogeneic chimeras are specifically tolerant of both donor and recipient, and fully reactive to third-party cells and tissue grafts, they fail to exhibit primary humoral immune responses (9) and have deficient cellular immune responses to certain intracellular pathogens (10).
Ildstad et al. (11) discovered that chimeras transplanted with mixed T-cell-depleted marrow (TCDM) from both allogeneic and syngeneic donors can fully reconstitute hematopoietic and immunologic function after supralethal TBI and do not express the immunological deficits observed after TBI plus fully allogeneic bone marrow. El-Badri and Good (12, 13) extended the research of Ildstad et al. by showing survival in high frequency with stable mixed chimerism and normally vigorous functioning immune systems in C57BL/6 (B6) mice transplanted with TCDM from both BALB/c allogeneic and B6 syngeneic donors that differ from each other over the entire MHC. Such chimeras lacked the immunodeficiencies that are regularly observed in fully allogeneic chimeras.
BXSB mice spontaneously develop a human lupus-like autoimmune disease and die from immune complex-mediated glomerulonephritis that is somewhat different in distribution and manifestations from renal diseases characteristic of mice of other autoimmune-prone strains (14). A mutant gene, Yaa, located in the Y chromosome of BXSB mice, was found to profoundly enhance autoimmune abnormalities in this strain (15, 16).
The present investigation was designed to test the possibility of developing BMT further to prevent autoimmune diseases and to reconstruct full immunity functions of irradiated mice at the same time by transplanting mixed TCDM from both allogeneic, autoimmune-resistant donor mice and syngeneic, autoimmune-prone donor mice into lethally irradiated BXSB recipients at once to correct the propensity to autoimmune disease and to avoid the annoying immunodeficiencies. If this were successful, it might suggest an important new approach to treatment of autoimmune diseases by BMT that accompany TBI and fully allogeneic marrow transplantation across the MHC barrier.
Lethally irradiated BXSB mice were transplanted with mixed TCDM cells from both allogeneic, autoimmune-resistant BALB/c donor mice and syngeneic, autoimmune-prone BXSB mice. Eight-week-old recipient BXSB mice transplanted with a mixture of fully allogeneic and syngeneic TCDM cells were found to have prolonged lifespans, lower levels of serum antibodies to double-stranded DNA (dsDNA), and higher numbers of primary-antibody-producing, plaque-forming cells (PFCs) than did mice transplanted with syngeneic BXSB TCDM cells. These mice also produced primary antibody responses significantly greater than those produced by mice transplanted with bone marrow from fully allogeneic donors that differed from recipients across the entire MHC. This transplantation of mixed TCDM also inhibited the development of autoimmune-associated glomerulonephritis in the BXSB recipient mice.
MATERIALS AND METHODS
Mice.
Seven- to eight-week-old male BXSB, BALB/c, and B6 mice were purchased from The Jackson Laboratory and were maintained in a pathogen-free environment.
BMT.
Bone marrow cells were harvested from the femurs and tibias of donors and depleted of T cells by complement-dependent cytotoxicity using purified anti-Thy-1.2 monoclonal antibody (PharMingen) and rabbit complement (Cedarlane Laboratories). Eight- to 10-week old male recipient BXSB mice were given 9.5 Gy of TBI (137Cs irradiation, 0.75 Gy/min) and reconstituted intravenously by BMT of TCDM in a standard fashion. Different donor TCDM cells were used in the following transplantation groups: group I, 15 × 106 allogeneic, autoimmune-resistant BALB/c TCDM cells plus 5 × 106 syngeneic, autoimmune-prone BXSB TCDM cells for [BALB/c + BXSB → BXSB] experimental group (20 recipients); group II, 15 × 106 allogeneic BALB/c TCDM cells plus 5 × 106 MHC-matched, autoimmune-resistant B6 TCDM cells for [BALB/c + B6 → BXSB] treatment control group (8 recipients); and group III, 15 × 106 syngeneic BXSB TCDM cells for [BXSB → BXSB] autoimmune-disease control group (7 recipients). [BALB/c → BXSB] and [B6 → BALB/c] (3 recipients each) served as fully allogeneic BMT controls. [BALB/c → BALB/c] (3 recipients) were used as the normal syngeneic BMT control. Untreated BXSB mice (8 mice) were also employed as a control. All studies using animals were conducted in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care, in compliance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Assay of Chimerism.
Single-cell suspensions of spleen cells from chimeric mice at the age of 52 weeks (44 weeks after BMT) were incubated with fluorescein-conjugated anti-Kb and fluorescein-conjugated anti-Kd monoclonal antibodies (PharMingen), and subpopulations of allogeneic donor cells (Kd-positive) and syngeneic cells (Kb-positive) were quantified by using flow cytometric analysis.
Histopathology.
Samples of kidneys were obtained at autopsy and fixed in 10% neutral formalin, and histological sections were stained with either the periodic acid–Schiff (PAS) reagent or with hematoxylin and eosin (HE). Glomerulonephritis was scored on a 0–4 scale based on the intensity and extent of histopathological changes. A grade of 0 was given to kidney without glomerular lesions; grade 1 consisted of minimal thickening of the mesangium; grade 2 contained noticeable increases in both mesangial and glomerular cellularity; grade 3 kidneys were characterized by the preceding conditions with superimposed inflammatory exudates and capsular adhesions; and grade 4 consisted of obliterated glomerular architecture in greater than 70% of glomeruli. Twenty glomeruli within one area were graded according to this classification and used to calculate the mean glomerular histopathological score for each mouse, and those scores were used to calculate mean scores for each experimental cohort.
Anti-Sheep Erythrocyte PFC Assay.
Primary antibody production was tested in BXSB BMT recipient mice as well as untreated control mice when the mice were 52 weeks old. Each treated or control mouse was injected intraperitoneally with 0.5 ml of 2% sheep red blood cells (SRBC). Five days later, the injected mice were killed, and single-cell suspensions of spleen cells were adjusted to a concentration of 5 × 106 cells per ml. A mixture of 500 μl of 0.5% agarose (Sigma) solution, 100 μl of spleen cell suspension, and 50 μl of 1% SRBC suspension was poured onto a precoated microscope slide. Dried slides were carefully inverted in a tray and incubated with guinea pig complement (Cappel). After being incubated at 37°C for 3 hr, hemolytic plaques were counted and expressed as number of plaques per 5 × 105 cells.
Assay of Anti-dsDNA Autoantibodies.
Serum antibodies specific for dsDNA were determined by using an enzyme-linked immunosorbent assay (ELISA) as described elsewhere (17). The serum was diluted 1:100 for the assay. The concentration of anti-dsDNA was determined by reading the absorbance at 410 nm.
Statistical Analysis.
Statistical significance was determined by either a log-rank Mantel–Haenszel test or Student’s t test. P values <0.05 were considered significant.
RESULTS
Longevity.
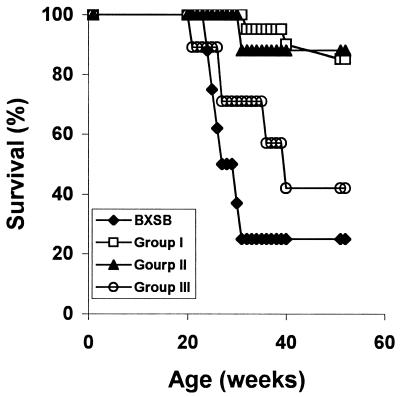
Within 44 weeks after transplantation (at an age of 52 weeks), 57% of the BXSB recipients of TCDM from autoimmune-prone BXSB donors had developed kidney disease and died of fulminant lethal glomerulonephritis (Fig. 1). In contrast, only 15% of the BXSB recipients of mixed BMT (transplanted with mixed TCDM from both autoimmune-resistant BALB/c donors and autoimmune-prone BXSB donors) had developed fatal renal disease in this interval, which is comparable to the percentage (12%) of the control group composed of BXSB recipients transplanted with mixed TCDM from two autoimmune-resistant allogeneic donors BALB/c MHC-mismatched plus MHC-matched B6 donors). Median survival age of recipients of BXSB TCDM was 40 weeks, whereas that of mice engrafted with mixed TCDM was >52 weeks, at which point the study was terminated. Median survival age of untreated BXSB mice was 33 weeks.
Figure 1.
Survival curves of male BXSB mice, exposed to 9.5 Gy of TBI (137Cs irradiation, 0.75 Gy/min), given intravenously TCDM cells from both BALB/c and BXSB (group I, n = 20, □), from both BALB/c and B6 (group II, n = 8, ▴), and from BXSB male donor mice (group III, n = 7, ○), when recipients were 8 to 10 weeks old. Untreated BXSB mice served as a control (n = 8, ⧫).
Chimeric Analysis.
As shown in Table 1, spleens from BXSB mice transplanted with allogeneic BALB/c TCDM cells were repopulated almost completely with cells of donor origin (H-2d). The percentages of cells of donor origin from allogeneic [BALB/c → BXSB] chimeras were comparable to those of cells of donor origin (H-2b) from [B6 → BALB/c] allogeneic chimeras (data not shown). The percentages of H-2d-positive cells (allogeneic origin) from BXSB mice transplanted with mixed TCDM were 42.0% from [BALB/c + BXSB → BXSB] chimeras and 54.9% from [BALB/c + B6 → BXSB] chimeric mice.
Table 1.
Chimerism of spleen cells in BXSB recipients transplanted with mixed TCDM
| Mice | Cell phenotype, %
|
|
|---|---|---|
| H-2d* | H-2b† | |
| Group I | 42.0 ± 14.3 | 48.8 ± 14.2 |
| Group II | 54.9 ± 10.6 | 37.7 ± 10.8 |
| BALB/c → BXSB | 92.6 ± 0.4 | 7.7 ± 1.9 |
Chimeric spleen cells were analyzed 44 weeks after transplantation. Group I, BALB/c + BXSB → BXSB; group II, BALB/c + B6 → BXSB. Results are mean ± SD.
Phenotype of BALB/c.
Phenotype of BXSB or B6.
Histopathology.
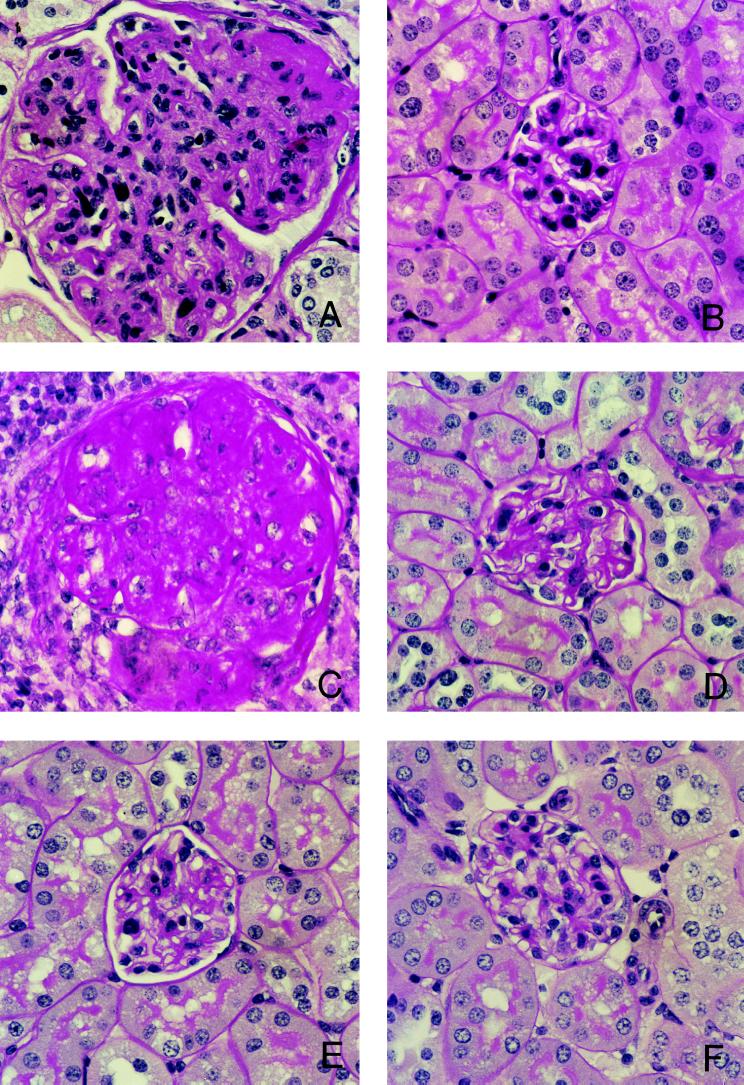
Glomerulonephritis within each kidney of chimeric mice or untreated BXSB mice at the age of 52 weeks was graded according to the intensity and extent of renal lesions. Mean glomerular lesion scores for each mouse were determined, and mean glomerular lesion scores for each experimental cohort were calculated and compared. Glomerular lesions of mice engrafted with mixed TCDM (from either BALB/c + BXSB or BALB/c + B6) were significantly less severe compared with those of recipients of BXSB TCDM, or BXSB untreated controls (Fig. 2). Mean glomerular lesion scores of [BALB/c + BXSB → BXSB] chimeric mice and those of [BALB/c + B6 → BXSB] chimeric mice were comparable (means of 0.85 and 0.61 with SD of ± 0.90 and ± 0.64, respectively). Mean glomerular lesion scores of BXSB control mice and those of the recipients of BXSB TCDM were comparable (means of 3.7 and 3.5 with SD of ± 0.58 and ± 0.71, respectively).
Figure 2.
Histopathology of kidneys from BXSB recipients transplanted with mixed TCDM. Representative histological appearance of glomerular lesions of kidneys from 52-week-old untreated mice or chimeras. (A) Untreated BXSB mice. (B) [BALB/c + BXSB → BXSB]. (C) [BXSB → BXSB]. (D) [BALB/c + B6 → BXSB]. (E) BALB/c mice. (F) [BALB/c → BXSB]. Note the striking marked differences in the size and histological abnormalities of glomeruli between BXSB recipients transplanted with mixed TCDM and those transplanted with BXSB TCDM cells. The tissues were stained with periodic acid–Schiff reagent. (×400.)
Primary Antibody Response.
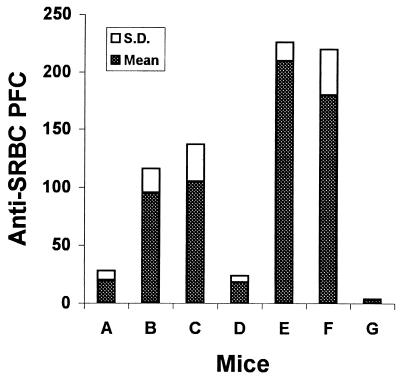
BXSB mice transplanted with TCDM were tested for ability to mount a primary antibody response against a cellular antigen, SRBC, in a PFC assay. As shown in Fig. 3, BXSB recipients transplanted with mixed TCDM (either with both BALB/c and BXSB or with both BALB/c and B6) in transplantation groups I and II, [BALB/c → BALB/c] normal syngeneic control group, and normal BALB/c mice produced a primary antibody response. However, inhibition of primary antibody formation was observed in BXSB untreated control mice, [BXSB → BXSB] transplants, and [BALB/c → BXSB] as well as [B6 → BALB/c] fully allogeneic chimeras.
Figure 3.
Primary anti-SRBC antibody response in BXSB recipients transplanted with TCDM cells. Bars: A, BXSB; B, [BALB/c + BXSB → BXSB]; C, [BALB/c + B6 → BXSB]; D, [BXSB → BXSB]; E, BALB/c; F, [BALB/c → BALB/c]; and G, [B6 → BALB/c]. PFCs are expressed per 5 × 105 spleen cells.
Anti-dsDNA Autoantibodies.
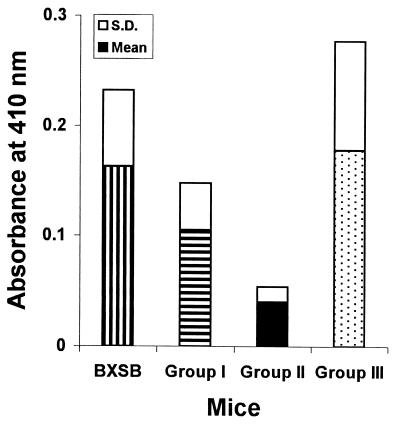
Serum levels of anti-dsDNA autoantibodies were determined for mice from each group of recipients as well as from the BXSB control group when mice were 52 weeks of age. As shown in Fig. 4, recipient BXSB mice transplanted with mixed TCDM (from either BALB/c + BXSB or BALB/c + B6) had lower levels of anti-dsDNA than [BXSB → BXSB] and BXSB mice.
Figure 4.
Serum levels of anti-dsDNA autoantibodies of TCDM recipient male BXSB mice when 52 weeks of age. Untreated BXSB mice served as control. Horizontally striped bar indicates group I, [BALB/c + BXSB → BXSB] chimeras. Solid bar indicates group II, [BALB/c + B6 → BXSB] chimeras. Stippled bar indicates group III, [BXSB → BXSB] transplants. Vertically striped bar indicates untreated BXSB mice.
DISCUSSION
The autoimmune diseases of humans are ultimately disabling and/or may cause premature death. The murine models of autoimmune diseases, such as that of BXSB mice, are invaluable in assessing the efficacy of various therapeutic modalities because they exhibit histologic and serologic similarities to human autoimmune diseases. Although it has been shown that BMT can prevent or cure autoimmune diseases in mice, the fully allogeneic chimeras produced in these experiments often experienced immunodeficiencies after TBI and BMT.
In the present study, we investigated whether creating stable mixed bone marrow chimerism by transplanting T-cell-purged allogeneic, autoimmune-resistant marrow, plus T-cell-purged syngeneic, autoimmune-prone marrow, would prevent autoimmune disease in BXSB mice. Mixed chimerism has advantages over fully allogeneic chimerism. Mixed chimeras have fully reconstituted normal immune functions, which are often missing in fully allogeneic chimeras. Mixed chimerism may also prevent fatal aplasia, because syngeneic bone marrow seldom fails to engraft. We found that autoimmune disease of BXSB mice could be prevented by transplantation of mixed TCDM from both BALB/c and BXSB mice. The development of renal histopathological lesions and the increase of serum anti-dsDNA level as observed in untreated BXSB mice and [BXSB → BXSB] transplants were abrogated in BXSB recipients transplanted with mixed TCDM from both BALB/c and BXSB donors and in BXSB mice reconstituted with mixed TCDM from both BALB/c and B6 mice. Median survival of [BALB/c + BXSB → BXSB] chimeric mice was comparable to that of [BALB/c + B6 → BXSB] and was significantly extended compared with that of BXSB recipients transplanted with BXSB TCDM or untreated BXSB mice. These findings showed that transplantation of mixed TCDM from allogeneic, autoimmune-resistant donor mice plus syngeneic, autoimmune-prone donor mice can be used to prevent murine autoimmune diseases, at least in BXSB mice.
BXSB recipients transplanted with mixed TCDM (from both allogeneic BALB/c plus syngeneic BXSB or allogeneic BALB/c plus MHC-matched B6 donor mice) produced primary antibodies against a cellular antigen, SRBC, in vivo, as did syngeneic transplants of [BALB/c → BALB/c] and untreated BALB/c mice. Fully allogeneic chimeras, such as [B6 → BALB/c] and [BALB/c → BXSB] did not produce primary antibodies against SRBC, and neither did [BXSB → BXSB] chimeric mice and untreated BXSB mice. Because all [BXSB → BXSB] chimeric mice or untreated BXSB mice at termination of the experiments experienced severe renal histopathological lesions and higher anti-dsDNA titer, they might not be able to mount a normal response to T-cell-dependent antigens such as SRBC. Transplantation of mixed TCDM from both allogeneic, autoimmune-resistant donor mice and syngeneic, autoimmune-prone donor mice reconstituted the immunity functions and prevented the development of autoimmune disease in the BXSB mice.
Allogeneic TCDM transplantation has been used as a means to treat a number of genetic and acquired diseases (5). Studies using mixed marrow from both allogeneic donor mice and syngeneic donor mice should be done to determine whether such treatment could be used to cure or palliate autoimmune diseases in autoimmune-prone mice and, at the same time, to reconstitute fully the immunity functions. Experiments are underway to see whether the same strategies used herein can be used to treat as well as prevent the autoimmune disease in BXSB mice.
Acknowledgments
We thank Ms. Tazim Verjee for her assistance in the preparation of this manuscript. This work was supported by National Institute on Aging Grant 05628–13 and a grant from the Children’s Research Institute by Mrs. Alice Goodyear.
ABBREVIATIONS
- TCDM
T-cell depleted marrow
- TBI
total-body irradiation
- BMT
bone marrow transplantation
- MHC
major histocompatibility complex
- B6
C57BL/6
- dsDNA
double-stranded DNA
- SRBC
sheep red blood cells
- PFC
plaque-forming cell
References
- 1.Ikehara S, Kawamura M, Takao F, Inaba M, Yasumiau R, Than S, Hisha H, Sugiura K, Koide Y, Yoshida T O, Ida T, Imura H, Good R A. Proc Natl Acad Sci USA. 1990;87:8341–8344. doi: 10.1073/pnas.87.21.8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikehara S, Kawamura M, Nishoka N, Nagata N, Good R A. In: New Strategies in Bone Marrow Transplantation. Champlin R E, Gale R P, editors. New York: Wiley–Liss; 1991. pp. 251–257. [Google Scholar]
- 3.Nakamura T, Ikehara S, Good R A, Inoue S, Sekita A, Furukawa F, Tanaka H, Maung O, Hamashima Y. Thymus. 1985;7:151–157. [PubMed] [Google Scholar]
- 4.Mizutani H, Engelman R W, Kinjoh K, Kurata Y, Ikehara S, Good R A. Blood. 1993;82:3091–3097. [PubMed] [Google Scholar]
- 5.Good R A. In: Bone Marrow Transplantation: Basic and Clinical Studies. Ikehara S, Takaku F, Good R A, editors. Tokyo: Springer; 1996. pp. 277–301. [Google Scholar]
- 6.Mizutani H, Engelman R W, Kurate Y, Ikehara S, Good R A. Blood. 1993;82:837–844. [PubMed] [Google Scholar]
- 7.Good R A, Kapoor N, Reisner Y. Cell Immunol. 1983;82:36–54. doi: 10.1016/0008-8749(83)90139-9. [DOI] [PubMed] [Google Scholar]
- 8.Good R A, Gatti R A, Hong R, Meuwissen H F. Exp Hematol. 1969;19:4–10. [Google Scholar]
- 9.Onoé K, Fernandez G, Good R A. J Exp Med. 1980;151:115–132. doi: 10.1084/jem.151.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onoé K, Good R A, Yamamoto K. J Immunol. 1985;136:4264–4269. [PubMed] [Google Scholar]
- 11.Ildstad S T, Wren S M, Bluestone J A, Barbieri S A, Sachs D H. J Exp Med. 1985;162:231–244. doi: 10.1084/jem.162.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Badri N, Good R A. Proc Soc Exp Biol Med. 1994;205:67–74. doi: 10.3181/00379727-205-43679. [DOI] [PubMed] [Google Scholar]
- 13.El-Badri N, Good R A. Proc Natl Acad Sci USA. 1993;90:6681–6685. doi: 10.1073/pnas.90.14.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theofilopoulos A N, McConahey P J, Izui S, Eisenberg R A, Pereira A B, Creighton W D. Clin Immunol Immunopathol. 1980;15:258–278. doi: 10.1016/0090-1229(80)90039-2. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg R A, Izui S, McConahey P J, Hang L M, Peters C J, Theofilopoulos A N, Dixon F J. J Immunol. 1980;125:1032–1036. [PubMed] [Google Scholar]
- 16.Hudgins C C, Steinberg R T, Klinman D M, Reeves M J P, Steinberg A D. J Immunol. 1985;134:3849–3854. [PubMed] [Google Scholar]
- 17.Sasaki T, Muryoi T, Sekiguchi Y, Tamate E, Yoshinaga K, Kitagawa Y. J Clin Immunol. 1985;5:246–253. doi: 10.1007/BF00929459. [DOI] [PubMed] [Google Scholar]