Abstract
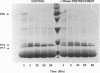
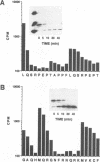
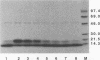
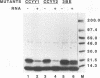
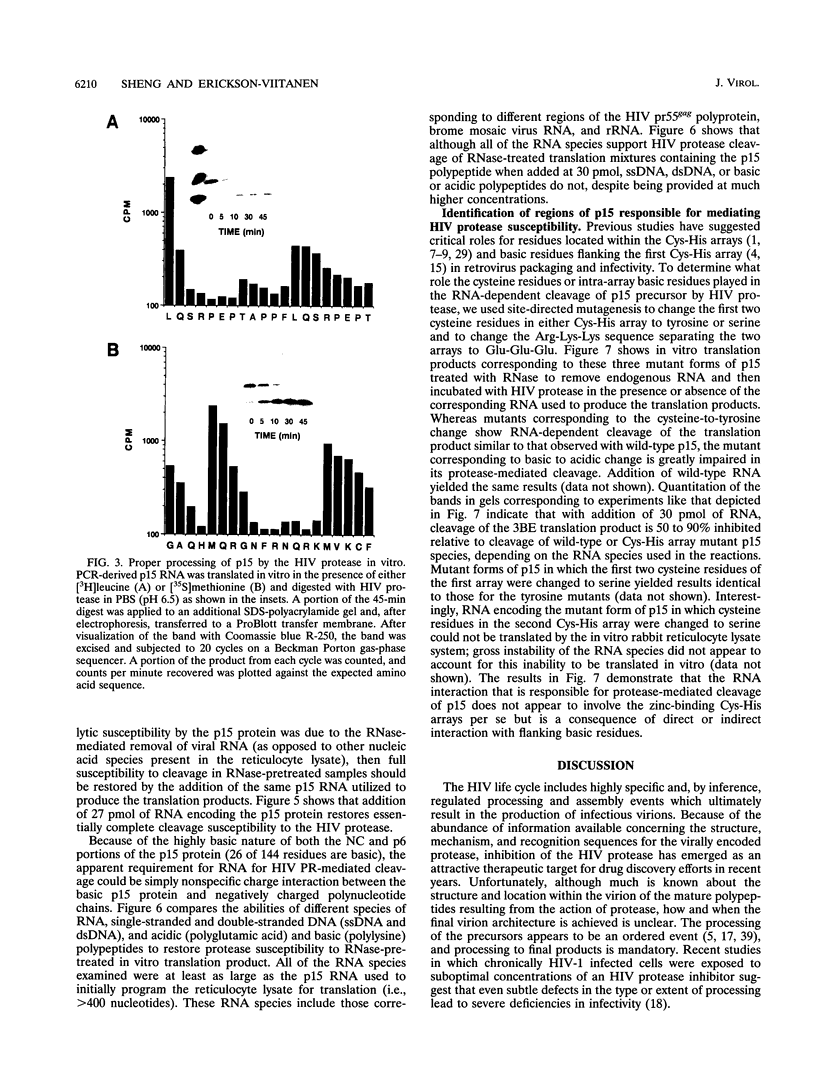
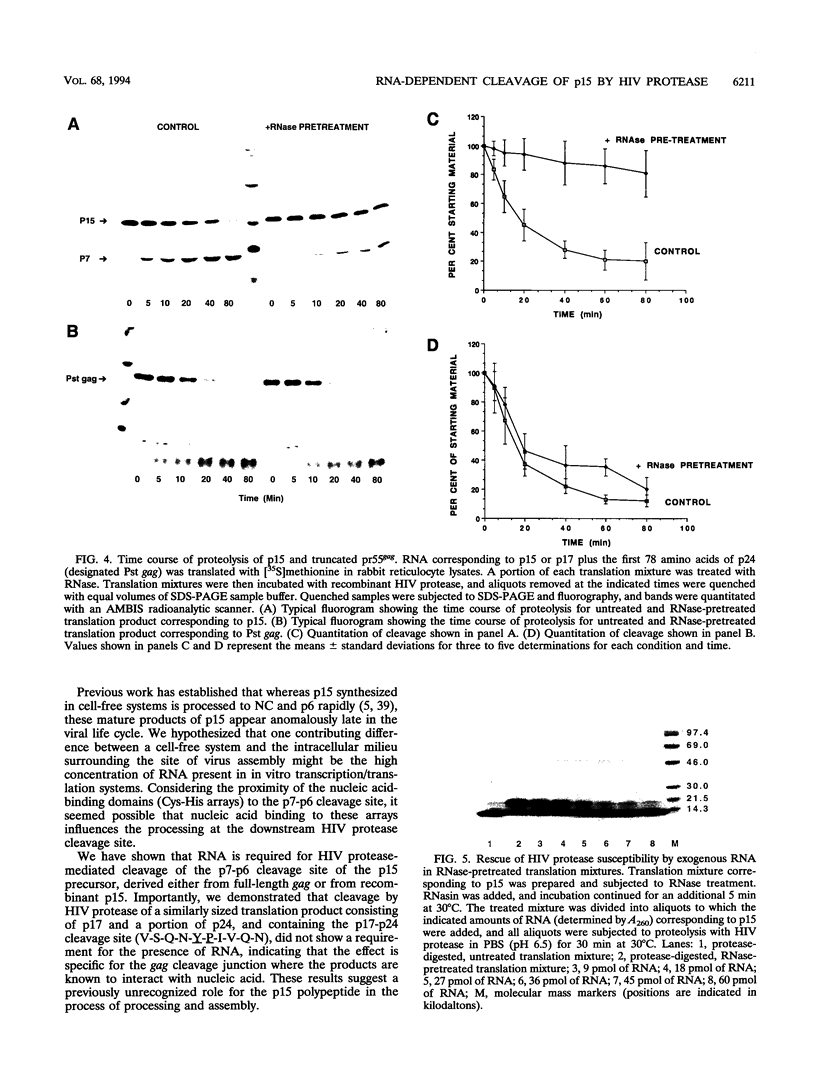
The human immunodeficiency virus (HIV) gag polyprotein is processed by the viral protease to yield the structural proteins of the virus. One of these structural proteins, p15, and its protease cleavage products, p7 and p6, are believed to be responsible for the viral RNA binding which is prerequisite for assembly of infectious virions. To better understand potential interactions between viral RNA, p15, and the HIV protease, we have synthesized p15 in an in vitro system and studied its processing by the viral protease. Using this system, we demonstrate that p15 synthesized in vitro is properly cleaved by the HIV protease in an RNA-dependent reaction. Mutation of cysteine residues in either zinc-binding domain of the p7 portion of p15 does not alter the RNA-dependent cleavage, but mutation of three basic residues located between the zinc-binding domains blocks HIV protease susceptibility. The results support a previously unrecognized role for the interaction of RNA and nucleocapsid-containing gag precursors that may have important consequences for virus assembly.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Young R. A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990 May;64(5):1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., McGowan M. H., Kettner C. A., Schloss J. V., Erickson-Viitanen S., Yin F. H. High-level synthesis of recombinant HIV-1 protease and the recovery of active enzyme from inclusion bodies. Gene. 1990 Mar 15;87(2):243–248. doi: 10.1016/0378-1119(90)90308-e. [DOI] [PubMed] [Google Scholar]
- Darke P. L., Nutt R. F., Brady S. F., Garsky V. M., Ciccarone T. M., Leu C. T., Lumma P. K., Freidinger R. M., Veber D. F., Sigal I. S. HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun. 1988 Oct 14;156(1):297–303. doi: 10.1016/s0006-291x(88)80839-8. [DOI] [PubMed] [Google Scholar]
- De Rocquigny H., Gabus C., Vincent A., Fournié-Zaluski M. C., Roques B., Darlix J. L. Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6472–6476. doi: 10.1073/pnas.89.14.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Viitanen S., Manfredi J., Viitanen P., Tribe D. E., Tritch R., Hutchison C. A., 3rd, Loeb D. D., Swanstrom R. Cleavage of HIV-1 gag polyprotein synthesized in vitro: sequential cleavage by the viral protease. AIDS Res Hum Retroviruses. 1989 Dec;5(6):577–591. doi: 10.1089/aid.1989.5.577. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Ozel M., Pauli G. Morphogenesis and morphology of HIV. Structure-function relations. Arch Virol. 1989;106(1-2):1–13. doi: 10.1007/BF01311033. [DOI] [PubMed] [Google Scholar]
- Gorelick R. J., Chabot D. J., Rein A., Henderson L. E., Arthur L. O. The two zinc fingers in the human immunodeficiency virus type 1 nucleocapsid protein are not functionally equivalent. J Virol. 1993 Jul;67(7):4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick R. J., Henderson L. E., Hanser J. P., Rein A. Point mutants of Moloney murine leukemia virus that fail to package viral RNA: evidence for specific RNA recognition by a "zinc finger-like" protein sequence. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8420–8424. doi: 10.1073/pnas.85.22.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick R. J., Nigida S. M., Jr, Bess J. W., Jr, Arthur L. O., Henderson L. E., Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990 Jul;64(7):3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Ikuta K., Zhang J. J., Morita C., Sano K., Komatsu M., Fujita H., Kato S., Nakai M. The budding of defective human immunodeficiency virus type 1 (HIV-1) particles from cell clones persistently infected with HIV-1. Arch Virol. 1990;111(1-2):87–101. doi: 10.1007/BF01310507. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Dorfman T., Sodroski J. G., Haseltine W. A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Bowers M. A., Sowder R. C., 2nd, Serabyn S. A., Johnson D. G., Bess J. W., Jr, Arthur L. O., Bryant D. K., Fenselau C. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processings, and complete amino acid sequences. J Virol. 1992 Apr;66(4):1856–1865. doi: 10.1128/jvi.66.4.1856-1865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Housset V., De Rocquigny H., Roques B. P., Darlix J. L. Basic amino acids flanking the zinc finger of Moloney murine leukemia virus nucleocapsid protein NCp10 are critical for virus infectivity. J Virol. 1993 May;67(5):2537–2545. doi: 10.1128/jvi.67.5.2537-2545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund S., Ofverstedt L. G., Nilsson A., Lundquist P., Gelderblom H., Ozel M., Skoglund U. Spatial visualization of the maturing HIV-1 core and its linkage to the envelope. AIDS Res Hum Retroviruses. 1992 Jan;8(1):1–7. doi: 10.1089/aid.1992.8.1. [DOI] [PubMed] [Google Scholar]
- Kaplan A. H., Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Zack J. A., Knigge M., Paul D. A., Kempf D. J., Norbeck D. W., Swanstrom R. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J Virol. 1993 Jul;67(7):4050–4055. doi: 10.1128/jvi.67.7.4050-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto T., Asanaka M., Kageyama S., Kurimura T., Nakajima K., Noto A., Tanaka H., Sato R. Budding process and maturation of human immunodeficiency virus examined by means of pre- and post-embedding immunocolloidal gold electron microscopy. J Electron Microsc (Tokyo) 1990;39(1):33–38. [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gu Z., Geleziunas R., Kleiman L., Wainberg M. A., Parniak M. A. Expression, purification, and RNA-binding properties of HIV-1 p15gag nucleocapsid protein. Protein Expr Purif. 1993 Aug;4(4):304–311. doi: 10.1006/prep.1993.1039. [DOI] [PubMed] [Google Scholar]
- Luban J., Goff S. P. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 gag polyprotein. J Virol. 1991 Jun;65(6):3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Goff S. P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989 Apr;63(4):1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., Housset V., de Billy G., Cornille F., Prats H., Roques B., Darlix J. L. Viral RNA annealing activities of the nucleocapsid protein of Moloney murine leukemia virus are zinc independent. Nucleic Acids Res. 1991 Jul 11;19(13):3533–3541. doi: 10.1093/nar/19.13.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. M., Copeland T. D., Oroszlan S. In situ processing of a retroviral nucleocapsid protein by the viral proteinase. Protein Eng. 1991 Aug;4(6):695–700. doi: 10.1093/protein/4.6.695. [DOI] [PubMed] [Google Scholar]
- Royer W. E., Jr, Love W. E., Fenderson F. F. Cooperative dimeric and tetrameric clam haemoglobins are novel assemblages of myoglobin folds. Nature. 1985 Jul 18;316(6025):277–280. doi: 10.1038/316277a0. [DOI] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Fields B. N. Characterization of a zinc blotting technique: evidence that a retroviral gag protein binds zinc. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4195–4199. doi: 10.1073/pnas.85.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South T. L., Blake P. R., Sowder R. C., 3rd, Arthur L. O., Henderson L. E., Summers M. F. The nucleocapsid protein isolated from HIV-1 particles binds zinc and forms retroviral-type zinc fingers. Biochemistry. 1990 Aug 28;29(34):7786–7789. doi: 10.1021/bi00486a002. [DOI] [PubMed] [Google Scholar]
- South T. L., Summers M. F. Zinc- and sequence-dependent binding to nucleic acids by the N-terminal zinc finger of the HIV-1 nucleocapsid protein: NMR structure of the complex with the Psi-site analog, dACGCC. Protein Sci. 1993 Jan;2(1):3–19. doi: 10.1002/pro.5560020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. F., Henderson L. E., Chance M. R., Bess J. W., Jr, South T. L., Blake P. R., Sagi I., Perez-Alvarado G., Sowder R. C., 3rd, Hare D. R. Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci. 1992 May;1(5):563–574. doi: 10.1002/pro.5560010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritch R. J., Cheng Y. E., Yin F. H., Erickson-Viitanen S. Mutagenesis of protease cleavage sites in the human immunodeficiency virus type 1 gag polyprotein. J Virol. 1991 Feb;65(2):922–930. doi: 10.1128/jvi.65.2.922-930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., Rahman R., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Immunological and chemical analysis of P6, the carboxyl-terminal fragment of HIV P15. AIDS Res Hum Retroviruses. 1987 Fall;3(3):253–264. doi: 10.1089/aid.1987.3.253. [DOI] [PubMed] [Google Scholar]
- Wondrak E. M., Louis J. M., de Rocquigny H., Chermann J. C., Roques B. P. The gag precursor contains a specific HIV-1 protease cleavage site between the NC (P7) and P1 proteins. FEBS Lett. 1993 Oct 25;333(1-2):21–24. doi: 10.1016/0014-5793(93)80367-4. [DOI] [PubMed] [Google Scholar]
- You J. C., McHenry C. S. HIV nucleocapsid protein. Expression in Escherichia coli, purification, and characterization. J Biol Chem. 1993 Aug 5;268(22):16519–16527. [PubMed] [Google Scholar]