Abstract
Cytomegalovirus is transmitted with blood and organs from seropositive individuals, although the particular leukocyte population harboring latent or persistent virus remains poorly characterized. Murine cytomegalovirus, tagged with the Escherichia coli lacZ gene, was used to identify cells in which virus replicates during acute infection of immunocompetent mice. Recombinant murine cytomegaloviruses, RM461, RM460, and RM427, were constructed to express beta-galactosidase under control of the human cytomegalovirus ie1/ie2 promoter/enhancer. The lacZ gene was inserted between the ie2 and sgg1 genes in RM461 and RM460, disrupting a 0.85-kb late transcript that was found to be dispensable for replication in cultured cells as well as for infection of mice. In BALB/c mice, lacZ-tagged and wild-type viruses exhibited a similar 50% lethal dose and all had the capacity to latently infect the spleen. Peripheral blood mononuclear phagocytes were the major infected leukocyte cell type, as demonstrated by the ability of infected cells to adhere to glass and to phagocytize latex beads; however, these cells did not exhibit typical monocyte markers. Plaque assay for virus and 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-Gal) staining of frozen sections of organs from infected mice revealed that the major target organs included the spleen, adrenal glands, liver, and salivary glands, although tissues as diverse as brown fat and lungs were also involved. Individual blue-staining cells were readily identified in all infected tissues. These studies identified a mononuclear phagocyte, possibly a macrophage or dendritic cell precursor, as the vehicle of virus dissemination during acute infection, and demonstrate the utility of using lacZ-tagged murine cytomegalovirus for tropism, pathogenesis, and latency studies.
Full text
PDF


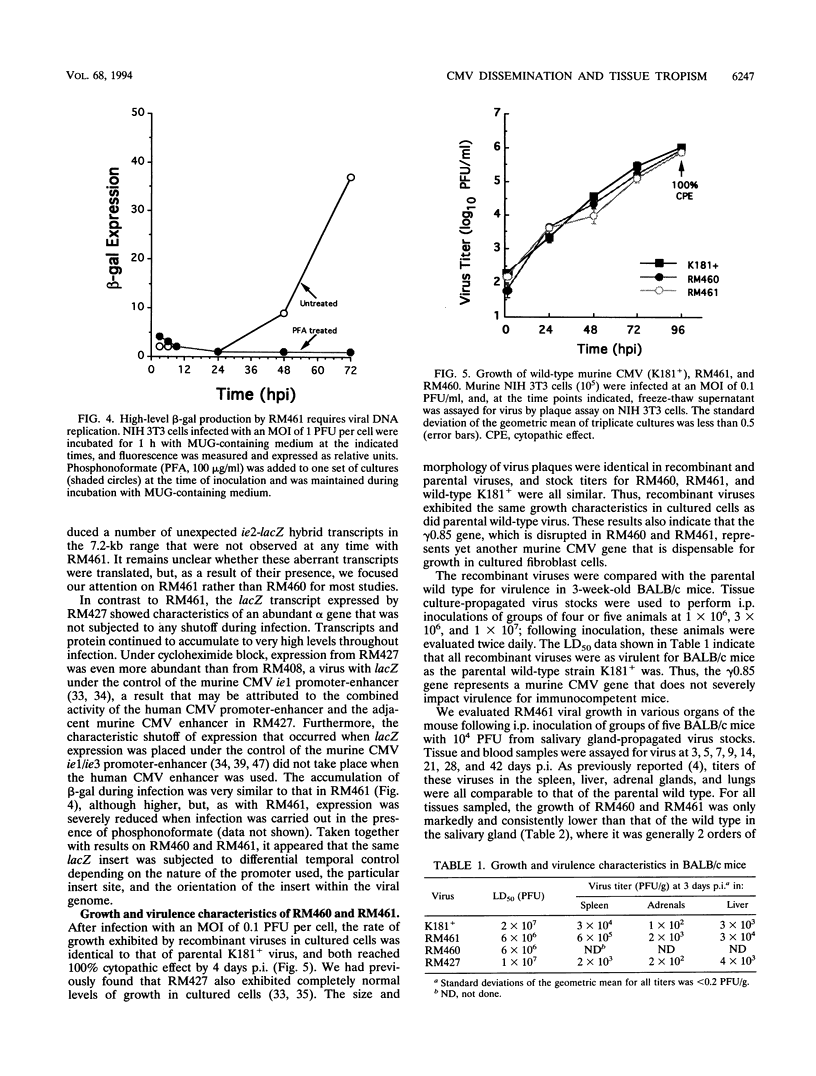
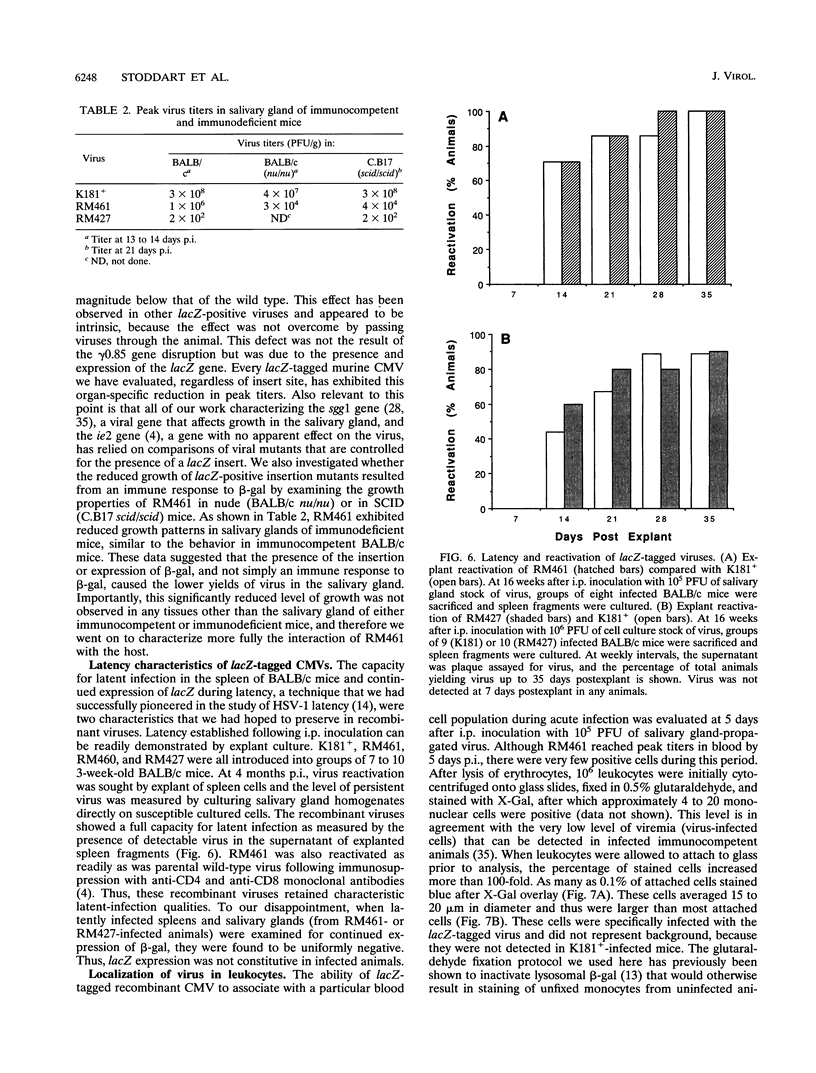
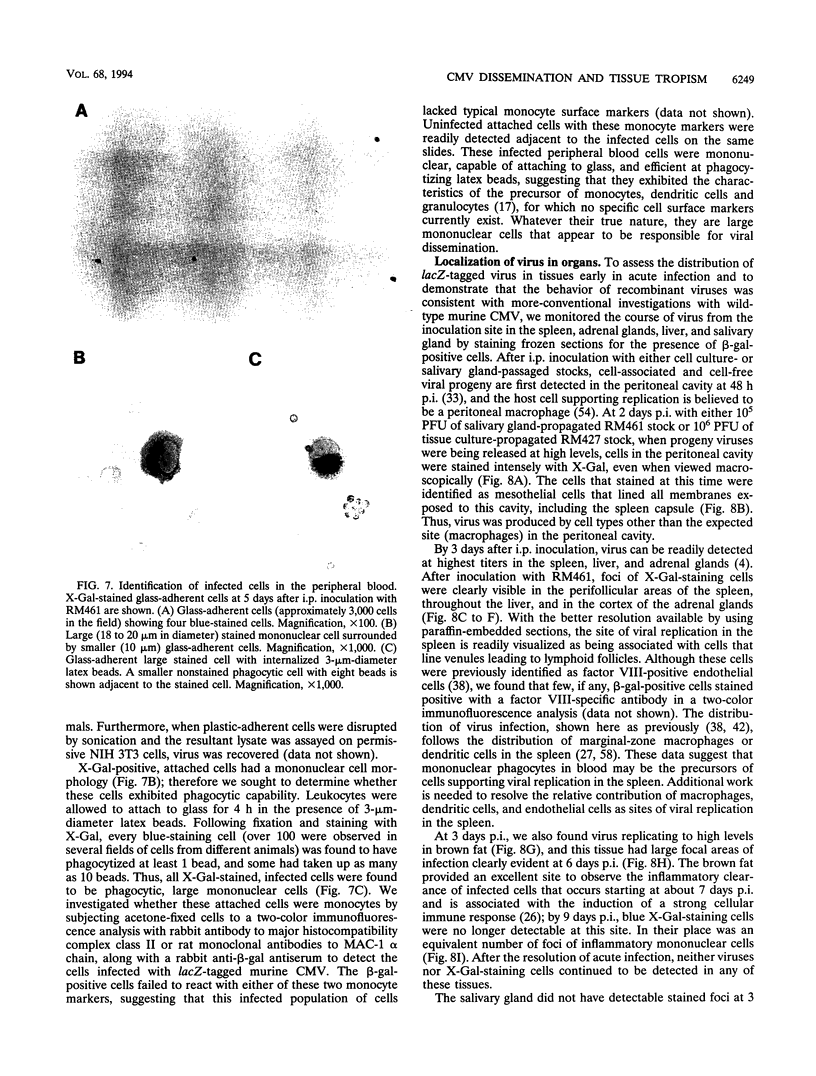
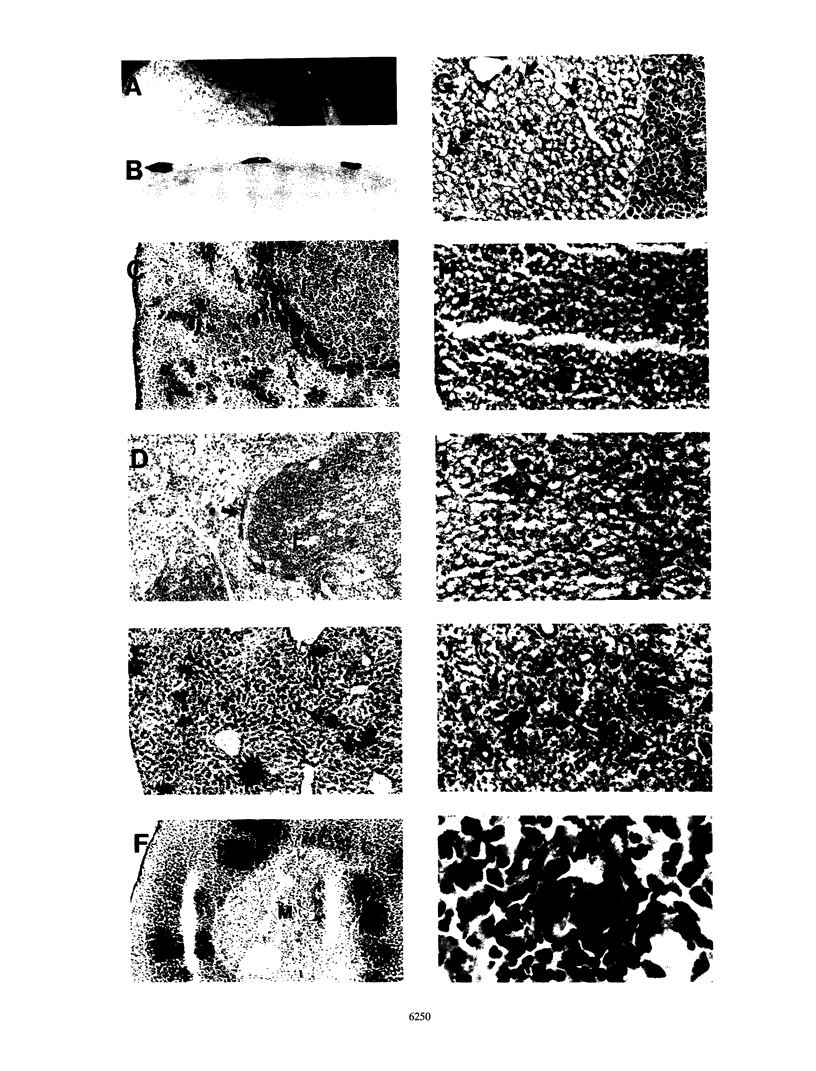



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale J. F., Jr, O'Neil M. E. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J Virol. 1989 Jun;63(6):2667–2673. doi: 10.1128/jvi.63.6.2667-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam A. R., Dutko F. J., Olding L. B., Oldstone M. B. Pathogenesis of murine cytomegalovirus infection: the macrophage as a permissive cell for cytomegalovirus infection, replication and latency. J Gen Virol. 1979 Aug;44(2):349–359. doi: 10.1099/0022-1317-44-2-349. [DOI] [PubMed] [Google Scholar]
- Cherrington J. M., Mocarski E. S. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J Virol. 1989 Mar;63(3):1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Lang D. J. Transmission and activation of cytomegalovirus with blood transfusion: a mouse model. J Infect Dis. 1977 May;135(5):841–845. doi: 10.1093/infdis/135.5.841. [DOI] [PubMed] [Google Scholar]
- Converse P. J., Hess A. D., Tutschka P. J., Santos G. W. Effect of cyclosporine on the response of normal human lymphocytes to cytomegalovirus in vitro. Infect Immun. 1983 Sep;41(3):1226–1233. doi: 10.1128/iai.41.3.1226-1233.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankner W. M., McCutchan J. A., Richman D. D., Hirata K., Spector S. A. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J Infect Dis. 1990 Jan;161(1):31–36. doi: 10.1093/infdis/161.1.31. [DOI] [PubMed] [Google Scholar]
- Dudding L. R., Garnett H. M. Interaction of strain AD169 and a clinical isolate of cytomegalovirus with peripheral monocytes: the effect of lipopolysaccharide stimulation. J Infect Dis. 1987 May;155(5):891–896. doi: 10.1093/infdis/155.5.891. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late gene expression: gamma genes are controlled by posttranscriptional events. J Virol. 1986 Mar;57(3):864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe A. P., Spaete R. R., Mocarski E. S. A cis-acting element within the 5' leader of a cytomegalovirus beta transcript determines kinetic class. Cell. 1986 Sep 12;46(6):865–872. doi: 10.1016/0092-8674(86)90068-1. [DOI] [PubMed] [Google Scholar]
- Grefte A., van der Giessen M., van Son W., The T. H. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J Infect Dis. 1993 Feb;167(2):270–277. doi: 10.1093/infdis/167.2.270. [DOI] [PubMed] [Google Scholar]
- Ho D. Y., Mocarski E. S. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988 Nov;167(1):279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Ho D. Y., Mocarski E. S. Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7596–7600. doi: 10.1073/pnas.86.19.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C. E., Schrier R., Ghazal P., Wiley C., Nelson J. A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991 Dec;65(12):6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Deguchi M., Hagi K., Yasumizu R., Ikehara S., Muramatsu S., Steinman R. M. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Naito M., Steinman R. M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993 Aug 1;178(2):479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Pack M. W., Aya H., Inaba M., Sudo T., Wolpe S., Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992 May 1;175(5):1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., Mutter W., Weiland F., Reddehase M. J., Koszinowski U. H. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989 Apr 1;169(4):1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C., Mar V. L. Spontaneous activation of latent cytomegalovirus from murine spleen explants. Role of lymphocytes and macrophages in release and replication of virus. J Clin Invest. 1982 Oct;70(4):762–768. doi: 10.1172/JCI110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Immediate-early genes of murine cytomegalovirus: location, transcripts, and translation products. J Virol. 1987 Feb;61(2):526–533. doi: 10.1128/jvi.61.2.526-533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987 Jun;61(6):1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984 Jun;50(3):784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman M. E., Henry S. C., Greene R. C., Brazy P. C., Klotman P. E., Hamilton J. D. Detection of mouse cytomegalovirus nucleic acid in latently infected mice by in vitro enzymatic amplification. J Infect Dis. 1990 Feb;161(2):220–225. doi: 10.1093/infdis/161.2.220. [DOI] [PubMed] [Google Scholar]
- Koszinowski U. H., Del Val M., Reddehase M. J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- Lathey J. L., Spector S. A. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J Virol. 1991 Nov;65(11):6371–6375. doi: 10.1128/jvi.65.11.6371-6375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late-gene expression: differential use of three start sites in the transcriptional activation of ICP36 gene expression. J Virol. 1989 Apr;63(4):1783–1791. doi: 10.1128/jvi.63.4.1783-1791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin P., Pavić I., Polić B., Jonjić S., Koszinowski U. H. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992 Apr;66(4):1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski J. P., Bruening E. E., Donahue R. E., Sellers S. E., Carter C., Young N. S., St Jeor S. Infection of mononucleated phagocytes with human cytomegalovirus. Virology. 1993 Aug;195(2):327–336. doi: 10.1006/viro.1993.1383. [DOI] [PubMed] [Google Scholar]
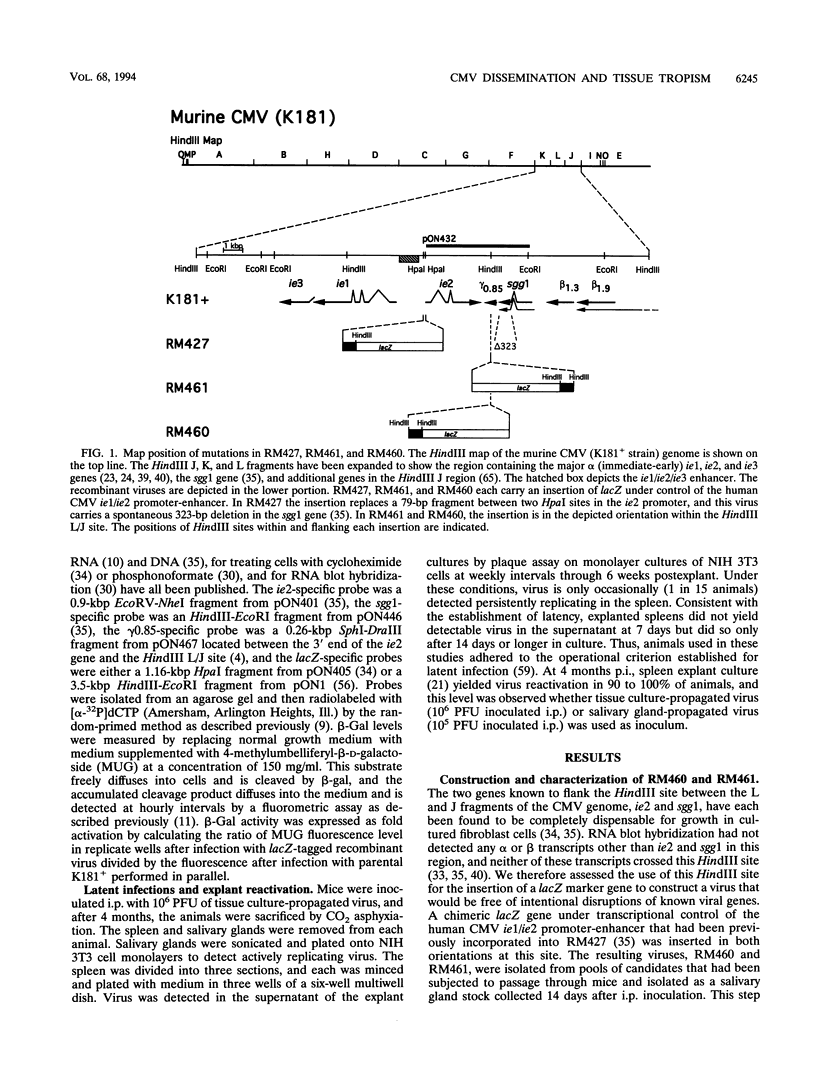
- Manning W. C., Mocarski E. S. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent alpha gene (ie2) is dispensable for growth. Virology. 1988 Dec;167(2):477–484. [PubMed] [Google Scholar]
- Manning W. C., Stoddart C. A., Lagenaur L. A., Abenes G. B., Mocarski E. S. Cytomegalovirus determinant of replication in salivary glands. J Virol. 1992 Jun;66(6):3794–3802. doi: 10.1128/jvi.66.6.3794-3802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. A., Marks J. R., Spector D. H. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith Strain). Virology. 1983 Aug;129(1):94–106. doi: 10.1016/0042-6822(83)90398-7. [DOI] [PubMed] [Google Scholar]
- Mercer J. A., Wiley C. A., Spector D. H. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J Virol. 1988 Mar;62(3):987–997. doi: 10.1128/jvi.62.3.987-997.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerle M., Bühler B., Keil G. M., Koszinowski U. H. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J Virol. 1992 Jan;66(1):27–36. doi: 10.1128/jvi.66.1.27-36.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerle M., Keil G. M., Koszinowski U. H. Structure and expression of murine cytomegalovirus immediate-early gene 2. J Virol. 1991 Mar;65(3):1638–1643. doi: 10.1128/jvi.65.3.1638-1643.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Gould J. Infection of salivary glands, kidneys, adrenals, ovaries and epithelia by murine cytomegalovirus. J Med Microbiol. 1979 Feb;12(1):113–122. doi: 10.1099/00222615-12-1-113. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Gould J. Splenic necrosis in mice infected with cytomegalovirus. J Infect Dis. 1978 May;137(5):587–591. doi: 10.1093/infdis/137.5.587. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Gould J. The role of macrophages in mice infected with murine cytomegalovirus. J Gen Virol. 1978 Oct;41(1):143–153. doi: 10.1099/0022-1317-41-1-143. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Bonyhadi M., Salimi S., McCune J. M., Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):104–108. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson D., Hackman R. C., Nelson J. A., Ward D. C., McDougall J. K. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984 May;15(5):430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Fibi M. R., Keil G. M., Koszinowski U. H. Late-phase expression of a murine cytomegalovirus immediate-early antigen recognized by cytolytic T lymphocytes. J Virol. 1986 Dec;60(3):1125–1129. doi: 10.1128/jvi.60.3.1125-1129.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Weiland F., Münch K., Jonjic S., Lüske A., Koszinowski U. H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985 Aug;55(2):264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman R. L., Quirk M. R., Jordan M. C. Disseminated cytomegalovirus infection. Molecular analysis of virus and leukocyte interactions in viremia. J Clin Invest. 1988 Jan;81(1):75–81. doi: 10.1172/JCI113313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley J. D., Biczak L., Forman S. J. Acute murine cytomegalovirus infection induces lethal hepatitis. J Infect Dis. 1993 Feb;167(2):264–269. doi: 10.1093/infdis/167.2.264. [DOI] [PubMed] [Google Scholar]
- Shanley J. D., Jordan M. C., Stevens J. G. Modification by adoptive humoral immunity of murine cytomegalovirus infection. J Infect Dis. 1981 Feb;143(2):231–237. doi: 10.1093/infdis/143.2.231. [DOI] [PubMed] [Google Scholar]
- Shanley J. D., Pesanti E. L. The relation of viral replication to interstitial pneumonitis in murine cytomegalovirus lung infection. J Infect Dis. 1985 Mar;151(3):454–458. doi: 10.1093/infdis/151.3.454. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent infections induced by herpes simplex viruses. Cancer Res. 1973 Jun;33(6):1399–1401. [PubMed] [Google Scholar]
- Stoddart C. A., Scott F. W. Isolation and identification of feline peritoneal macrophages for in vitro studies of coronavirus-macrophage interactions. J Leukoc Biol. 1988 Nov;44(5):319–328. doi: 10.1002/jlb.44.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Chiba S., Abo W., Chiba Y., Nakao T. Cytomegalovirus-specific lymphocyte transformations in subjects of different ages with primary immunodeficiency. Infect Immun. 1980 Apr;28(1):49–53. doi: 10.1128/iai.28.1.49-53.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Hayhurst G. P., Sissons J. G., Sinclair J. H. Polymorphonuclear cells are not sites of persistence of human cytomegalovirus in healthy individuals. J Gen Virol. 1993 Feb;74(Pt 2):265–268. doi: 10.1099/0022-1317-74-2-265. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. J., Craighead J. E. Infection of adult mouse macrophages in vitro with cytomegalovirus. Proc Soc Exp Biol Med. 1968 Dec;129(3):690–694. doi: 10.3181/00379727-129-33399. [DOI] [PubMed] [Google Scholar]
- Turtinen L. W., Saltzman R., Jordan M. C., Haase A. T. Interactions of human cytomegalovirus with leukocytes in vivo: analysis by in situ hybridization. Microb Pathog. 1987 Oct;3(4):287–297. doi: 10.1016/0882-4010(87)90062-3. [DOI] [PubMed] [Google Scholar]
- Vieira J., Farrell H. E., Rawlinson W. D., Mocarski E. S. Genes in the HindIII J fragment of the murine cytomegalovirus genome are dispensable for growth in cultured cells: insertion mutagenesis with a lacZ/gpt cassette. J Virol. 1994 Aug;68(8):4837–4846. doi: 10.1128/jvi.68.8.4837-4846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Howell C. L., Miller M. J., Mickey R., Martin W. J., Lin C. H., Gale R. P. Cytomegalovirus infections associated with leukocyte transfusions. Ann Intern Med. 1980 Nov;93(5):671–675. doi: 10.7326/0003-4819-93-5-671. [DOI] [PubMed] [Google Scholar]