Abstract
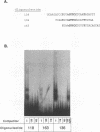
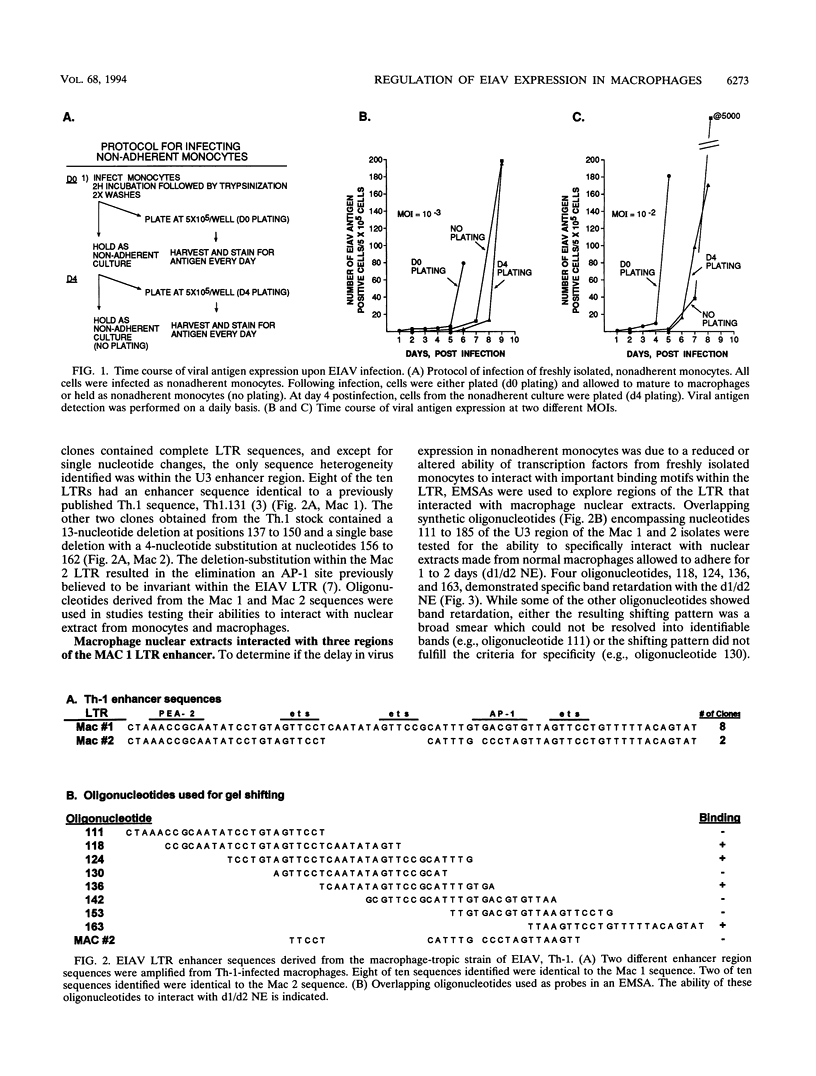
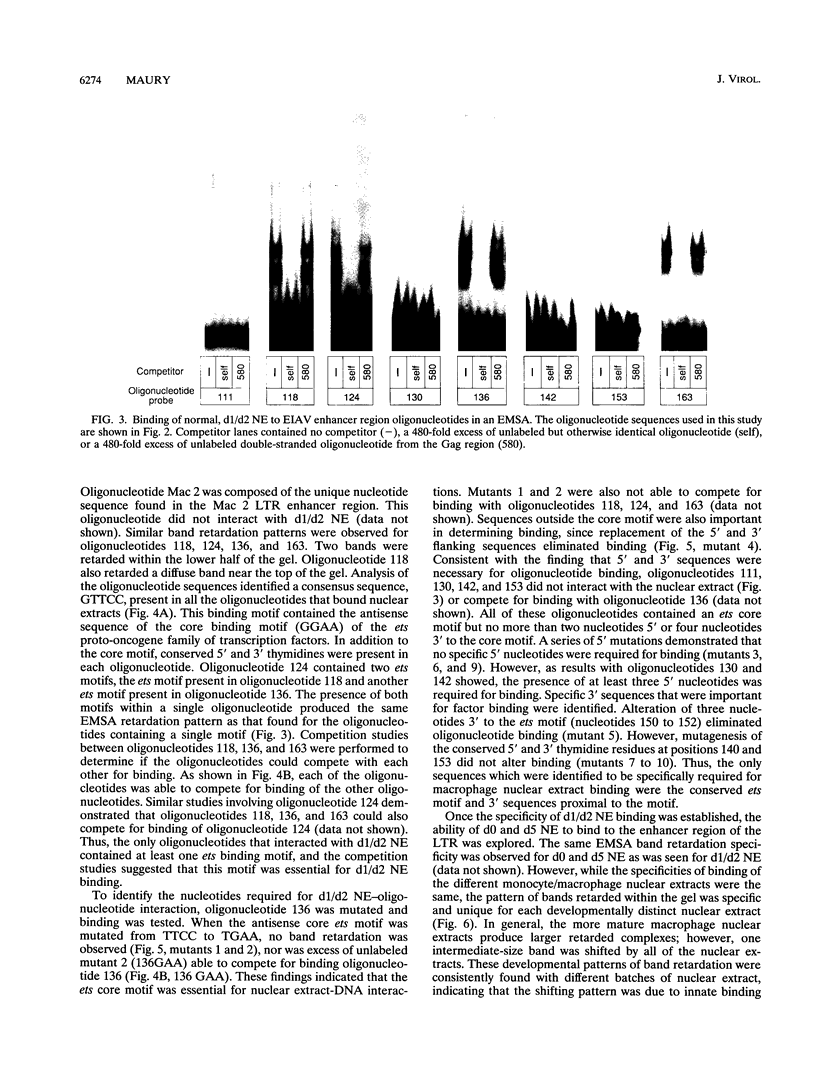
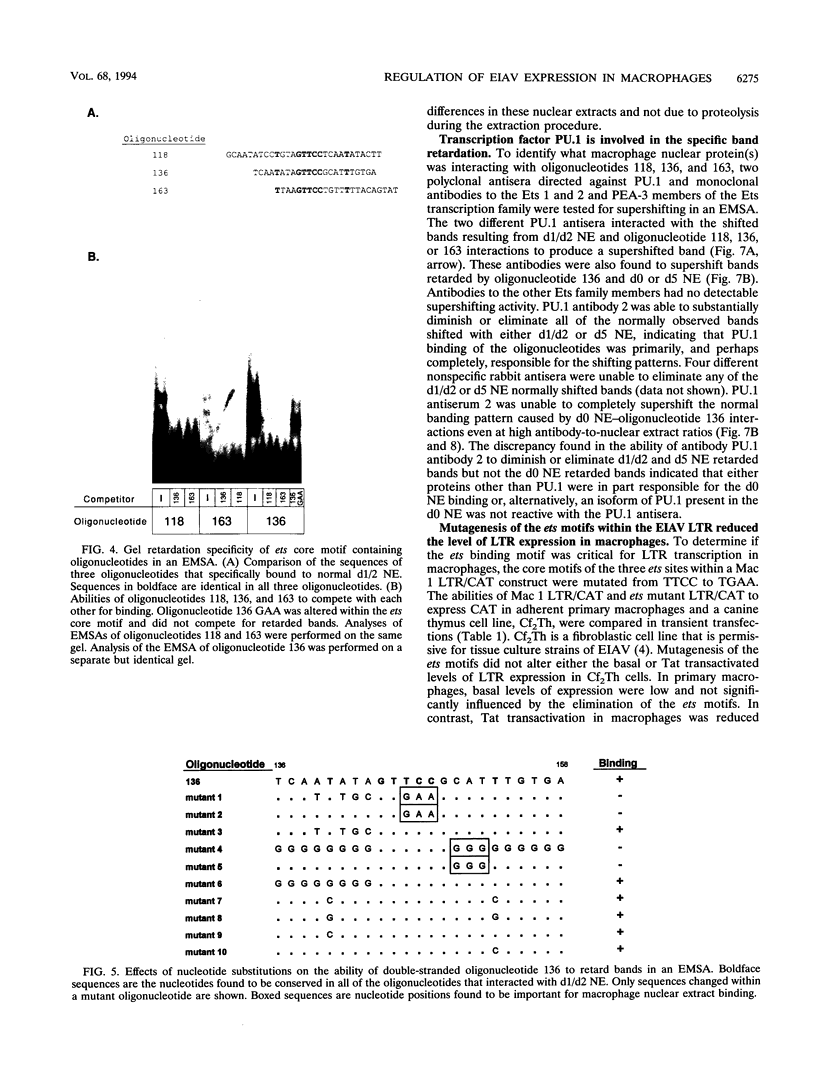
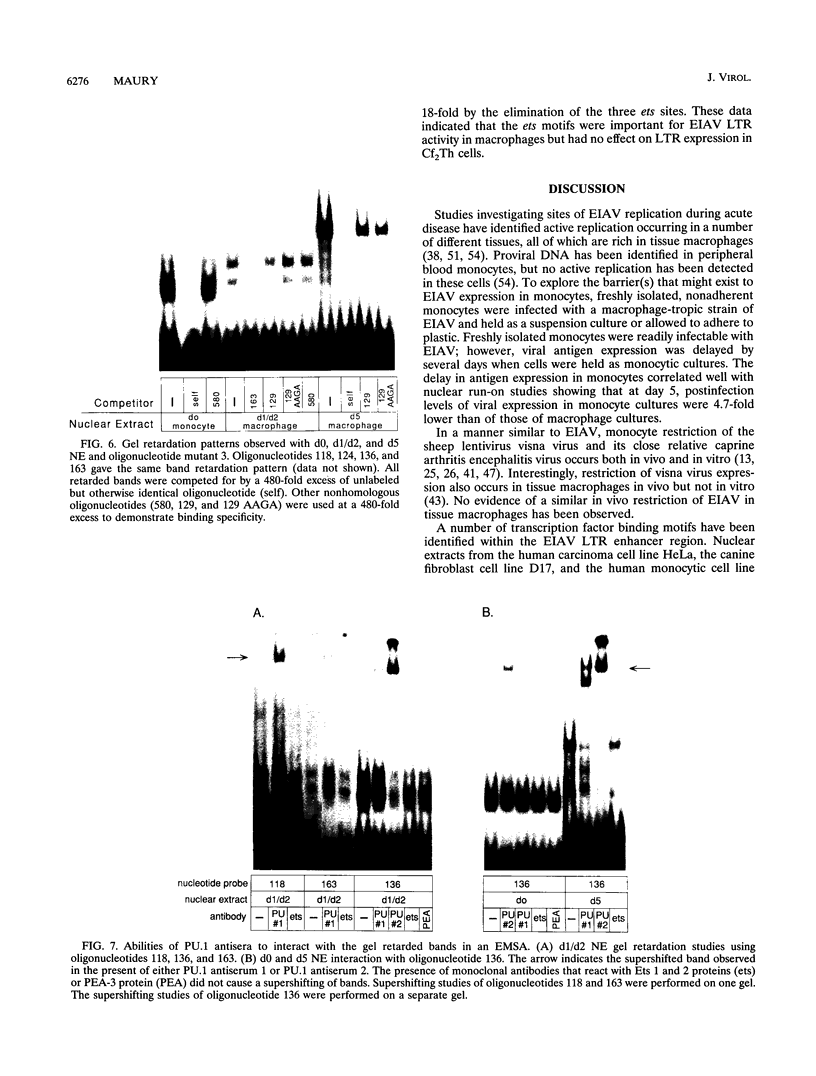
In vivo, equine infectious anemia virus (EIAV) replicates in tissues rich in macrophages, and it is widely believed that the tissue macrophage is the principal, if not sole, cell within the host that replicates virus. No viral replication has been detected in circulating peripheral blood monocytes. However, proviral DNA can be detected in these cells, and monocytes may serve as a reservoir for the virus. In this study, an in vitro model was developed to clarify the role of monocyte maturation in regulating EIAV expression. Freshly isolated, nonadherent equine peripheral blood monocytes were infected with a macrophage-tropic strain of EIAV, and expression of EIAV was monitored in cells held as nonadherent monocytes and cells allowed to adhere and differentiate into macrophages. A 2- to 3-day delay in viral antigen expression was observed in the nonadherent cells. This restriction of viral expression in monocytes was supported by nuclear run-on studies demonstrating that on day 5 postinfection, the level of actively transcribed viral messages was 4.7-fold lower in monocyte cultures than in macrophage cultures. Electrophoretic mobility shift assays identified three regions of the U3 enhancer that interacted with nuclear extracts from normal equine macrophages. Each region contained the core binding motif of a family of transcription factors that includes the product of the proto-oncogene ets. Antibodies to the Ets family member PU.1 caused a supershifting of retarded bands in an electrophoretic mobility shift assay. Transfection studies of ets motif mutants demonstrated that the U3 ets sites were important in the regulation of EIAV transcription in macrophages. Interactions between the ets motif and nuclear extracts from freshly isolated, nonadherent monocytes, macrophages adherent for 1 or 2 days, or macrophages adherent for 5 days gave different patterns of retarded bands, although the binding specificities were similar with all three extracts. The different complexes formed by monocyte and macrophage nuclear extracts may explain the enhanced ability of mature macrophages to support EIAV expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Pomerantz R. J. Human immunodeficiency virus type I provirus is demonstrated in peripheral blood monocytes in vivo: a study utilizing an in situ polymerase chain reaction. AIDS Res Hum Retroviruses. 1993 Jan;9(1):69–76. doi: 10.1089/aid.1993.9.69. [DOI] [PubMed] [Google Scholar]
- Carpenter S., Alexandersen S., Long M. J., Perryman S., Chesebro B. Identification of a hypervariable region in the long terminal repeat of equine infectious anemia virus. J Virol. 1991 Mar;65(3):1605–1610. doi: 10.1128/jvi.65.3.1605-1610.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Chesebro B. Change in host cell tropism associated with in vitro replication of equine infectious anemia virus. J Virol. 1989 Jun;63(6):2492–2496. doi: 10.1128/jvi.63.6.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M., Derse D. Physical and functional characterization of transcriptional control elements in the equine infectious anemia virus promoter. J Virol. 1993 Apr;67(4):2064–2074. doi: 10.1128/jvi.67.4.2064-2074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M., Derse D. The PU.1/Spi-1 proto-oncogene is a transcriptional regulator of a lentivirus promoter. J Virol. 1993 Jul;67(7):3885–3890. doi: 10.1128/jvi.67.7.3885-3890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M., Kirkland M., Derse D. Protein interactions with DNA elements in variant equine infectious anemia virus enhancers and their impact on transcriptional activity. J Virol. 1993 Nov;67(11):6586–6595. doi: 10.1128/jvi.67.11.6586-6595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., McGuire T. C. Equine infectious anemia virus: immunopathogenesis and persistence. Rev Infect Dis. 1985 Jan-Feb;7(1):83–88. doi: 10.1093/clinids/7.1.83. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Nishio J., Perryman S., Cann A., O'Brien W., Chen I. S., Wehrly K. Identification of human immunodeficiency virus envelope gene sequences influencing viral entry into CD4-positive HeLa cells, T-leukemia cells, and macrophages. J Virol. 1991 Nov;65(11):5782–5789. doi: 10.1128/jvi.65.11.5782-5789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J Virol. 1988 Oct;62(10):3779–3788. doi: 10.1128/jvi.62.10.3779-3788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clabough D. L., Gebhard D., Flaherty M. T., Whetter L. E., Perry S. T., Coggins L., Fuller F. J. Immune-mediated thrombocytopenia in horses infected with equine infectious anemia virus. J Virol. 1991 Nov;65(11):6242–6251. doi: 10.1128/jvi.65.11.6242-6251.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark N. M., Smith M. J., Hilfinger J. M., Markovitz D. M. Activation of the human T-cell leukemia virus type I enhancer is mediated by binding sites for Elf-1 and the pets factor. J Virol. 1993 Sep;67(9):5522–5528. doi: 10.1128/jvi.67.9.5522-5528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. E., Gabuzda D. H., Gdovin S. L. Cell type specific and viral regulation of visna virus gene expression. Virus Res. 1990 Jun;16(2):175–183. doi: 10.1016/0168-1702(90)90021-3. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Dorn P., DaSilva L., Martarano L., Derse D. Equine infectious anemia virus tat: insights into the structure, function, and evolution of lentivirus trans-activator proteins. J Virol. 1990 Apr;64(4):1616–1624. doi: 10.1128/jvi.64.4.1616-1624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow S. W., Poss M. L., Hoover E. A. Feline immunodeficiency virus: a neurotropic lentivirus. J Acquir Immune Defic Syndr. 1990;3(7):658–668. [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Beneke J., Till M., Wolinsky S., Ribas J. L., Burke A., Haase A. T. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English R. V., Johnson C. M., Gebhard D. H., Tompkins M. B. In vivo lymphocyte tropism of feline immunodeficiency virus. J Virol. 1993 Sep;67(9):5175–5186. doi: 10.1128/jvi.67.9.5175-5186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Husayni H., Turpin J. A., Skillman D., Kalter D. C., Orenstein J. M., Hoover D. L., Meltzer M. S. Macrophage-HIV interaction: viral isolation and target cell tropism. AIDS. 1990 Mar;4(3):221–228. [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grove M., Plumb M. C/EBP, NF-kappa B, and c-Ets family members and transcriptional regulation of the cell-specific and inducible macrophage inflammatory protein 1 alpha immediate-early gene. Mol Cell Biol. 1993 Sep;13(9):5276–5289. doi: 10.1128/mcb.13.9.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gégonne A., Bosselut R., Bailly R. A., Ghysdael J. Synergistic activation of the HTLV1 LTR Ets-responsive region by transcription factors Ets1 and Sp1. EMBO J. 1993 Mar;12(3):1169–1178. doi: 10.1002/j.1460-2075.1993.tb05758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V. M., Zack P. M., Vogel A. P., Johnson P. R. Simian immunodeficiency virus infection of macaques: end-stage disease is characterized by widespread distribution of proviral DNA in tissues. J Infect Dis. 1991 May;163(5):976–988. doi: 10.1093/infdis/163.5.976. [DOI] [PubMed] [Google Scholar]
- Issel C. J., Coggins L. Equine infectious anemia: current knowledge. J Am Vet Med Assoc. 1979 Apr 1;174(7):727–733. [PubMed] [Google Scholar]
- Kazazi F., Mathijs J. M., Foley P., Cunningham A. L. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J Gen Virol. 1989 Oct;70(Pt 10):2661–2672. doi: 10.1099/0022-1317-70-10-2661. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Maury W. J., Carpenter S., Graves K., Chesebro B. Cellular and viral specificity of equine infectious anemia virus Tat transactivation. Virology. 1994 May 1;200(2):632–642. doi: 10.1006/viro.1994.1226. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B. Immunofluorescent localization of equine infectious anemia virus in tissue. Am J Pathol. 1971 Feb;62(2):283–294. [PMC free article] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Kodama T., Desrosiers R. C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992 Apr;66(4):2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses A. V., Ibanez C., Gaynor R., Ghazal P., Nelson J. A. Differential role of long terminal repeat control elements for the regulation of basal and Tat-mediated transcription of the human immunodeficiency virus in stimulated and unstimulated primary human macrophages. J Virol. 1994 Jan;68(1):298–307. doi: 10.1128/jvi.68.1.298-307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Zink M. C. Role of macrophages in lentivirus infections. Adv Vet Sci Comp Med. 1988;32:129–148. doi: 10.1016/b978-0-12-039232-2.50009-8. [DOI] [PubMed] [Google Scholar]
- Onuma M., Koomoto E., Furuyama H., Yasutomi Y., Taniyama H., Iwai H., Kawakami Y. Infection and dysfunction of monocytes induced by experimental inoculation of calves with bovine immunodeficiency-like virus. J Acquir Immune Defic Syndr. 1992 Oct;5(10):1009–1015. [PubMed] [Google Scholar]
- Pahl H. L., Scheibe R. J., Zhang D. E., Chen H. M., Galson D. L., Maki R. A., Tenen D. G. The proto-oncogene PU.1 regulates expression of the myeloid-specific CD11b promoter. J Biol Chem. 1993 Mar 5;268(7):5014–5020. [PubMed] [Google Scholar]
- Pasco D. S., Boyum K. W., Merchant S. N., Chalberg S. C., Fagan J. B. Transcriptional and post-transcriptional regulation of the genes encoding cytochromes P-450c and P-450d in vivo and in primary hepatocyte cultures. J Biol Chem. 1988 Jun 25;263(18):8671–8676. [PubMed] [Google Scholar]
- Peluso R., Haase A., Stowring L., Edwards M., Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985 Nov;147(1):231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol. 1992 Jan;12(1):368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser H. M., Wotton D., Gegonne A., Ghysdael J., Wang S., Speck N. A., Owen M. J. A phorbol ester response element within the human T-cell receptor beta-chain enhancer. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9934–9938. doi: 10.1073/pnas.89.20.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Holbrook N., Levens D. Binding of a cellular protein to the gibbon ape leukemia virus enhancer. Mol Cell Biol. 1987 Aug;7(8):2735–2744. doi: 10.1128/mcb.7.8.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Lequarre A. S., Casey J. W., Lahn S., Stephens R. M., Edwards J. Viral DNA in horses infected with equine infectious anemia virus. J Virol. 1989 Dec;63(12):5194–5200. doi: 10.1128/jvi.63.12.5194-5200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera R. R., Stuiver M. H., Steenbergen R., Murre C. Ets proteins: new factors that regulate immunoglobulin heavy-chain gene expression. Mol Cell Biol. 1993 Nov;13(11):7163–7169. doi: 10.1128/mcb.13.11.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S., Paul R., Gliniak B. C., Kabat D. Role of the PU.1 transcription factor in controlling differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1992 Jul;12(7):2967–2975. doi: 10.1128/mcb.12.7.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon D. C., Perry S. T., Coggins L., Fuller F. J. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J Virol. 1992 Oct;66(10):5906–5913. doi: 10.1128/jvi.66.10.5906-5913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyosaki T., Miyazawa T., Furuya T., Tomonaga K., Shin Y. S., Okita M., Kawaguchi Y., Kai C., Mori S., Mikami T. Localization of the viral antigen of feline immunodeficiency virus in the lymph nodes of cats at the early stage of infection. Arch Virol. 1993;131(3-4):335–347. doi: 10.1007/BF01378636. [DOI] [PubMed] [Google Scholar]
- Unger R. E., Marthas M. L., Lackner A. A., Pratt-Lowe E., Lohman B. L., Van Rompay K., Luciw P. A. Detection of simian immunodeficiency virus DNA in macrophages from infected rhesus macaques. J Med Primatol. 1992 Feb-May;21(2-3):74–81. [PubMed] [Google Scholar]
- Walker J. M., Bowen W. D., Patrick S. L., Williams W. E., Mascarella S. W., Bai X., Carroll F. I. A comparison of (-)-deoxybenzomorphans devoid of opiate activity with their dextrorotatory phenolic counterparts suggests role of sigma 2 receptors in motor function. Eur J Pharmacol. 1993 Jan 26;231(1):61–68. doi: 10.1016/0014-2999(93)90684-a. [DOI] [PubMed] [Google Scholar]
- Wotton D., Ghysdael J., Wang S., Speck N. A., Owen M. J. Cooperative binding of Ets-1 and core binding factor to DNA. Mol Cell Biol. 1994 Jan;14(1):840–850. doi: 10.1128/mcb.14.1.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. E., Hetherington C. J., Chen H. M., Tenen D. G. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994 Jan;14(1):373–381. doi: 10.1128/mcb.14.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]