Abstract
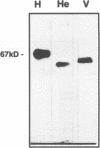
Two cellular proteins, membrane cofactor protein (MCP) and moesin, were reported recently to be functionally associated with the initiation of a measles virus infection. We have analyzed the interaction of measles virus with cell surface proteins, using an overlay binding assay with cellular proteins immobilized on nitrocellulose. Among surface-biotinylated proteins from a human rectal tumor cell line (HRT), measles virus was able to bind only to a 67-kDa protein that was identified as MCP. The virus recognized different isoforms of MCP expressed from human (HRT and HeLa) and simian (Vero) cell lines. The binding of measles virus to MCP was abolished after cleavage of the disulfide bonds by reducing agents as well as after enzymatic release of N-linked oligosaccharides. By contrast, removal of sialic acid or O-linked oligosaccharides did not affect the recognition of MCP measles virus. These data indicate that the receptor determinant of MCP is dependent on a conformation of the protein that is maintained by disulfide bonds and N-glycans present in the complement binding domains. Our results are consistent with a role of MCP as primary attachment site for measles virus in the initial stage of an infection. The functional relationship between MCP and moesin in a measles virus infection is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. M., Brown M. C., Nunge M., Krych M., Atkinson J. P. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991 Nov 1;147(9):3005–3011. [PubMed] [Google Scholar]
- Ballard L. L., Bora N. S., Yu G. H., Atkinson J. P. Biochemical characterization of membrane cofactor protein of the complement system. J Immunol. 1988 Dec 1;141(11):3923–3929. [PubMed] [Google Scholar]
- Ballard L., Seya T., Teckman J., Lublin D. M., Atkinson J. P. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45-70). J Immunol. 1987 Jun 1;138(11):3850–3855. [PubMed] [Google Scholar]
- Bhat S., Spitalnik S. L., Gonzalez-Scarano F., Silberberg D. H. Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7131–7134. doi: 10.1073/pnas.88.16.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut C., Krust B., Jacotot E., Hovanessian A. G. T cell activation antigen, CD26, as a cofactor for entry of HIV in CD4+ cells. Science. 1993 Dec 24;262(5142):2045–2050. doi: 10.1126/science.7903479. [DOI] [PubMed] [Google Scholar]
- Clayson E. T., Brando L. V., Compans R. W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989 May;63(5):2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dunster L. M., Schneider-Schaulies J., Löffler S., Lankes W., Schwartz-Albiez R., Lottspeich F., ter Meulen V. Moesin: a cell membrane protein linked with susceptibility to measles virus infection. Virology. 1994 Jan;198(1):265–274. doi: 10.1006/viro.1994.1029. [DOI] [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Harrowe G., Sudduth-Klinger J., Payan D. G. Measles virus-substance P receptor interaction: Jurkat lymphocytes transfected with substance P receptor cDNA enhance measles virus fusion and replication. Cell Mol Neurobiol. 1992 Oct;12(5):397–409. doi: 10.1007/BF00711541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Krah D. L. Characterization of octyl glucoside-solubilized cell membrane receptors for binding measles virus. Virology. 1989 Sep;172(1):386–390. doi: 10.1016/0042-6822(89)90147-5. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Lankes W. T., Furthmayr H. Moesin: a member of the protein 4.1-talin-ezrin family of proteins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8297–8301. doi: 10.1073/pnas.88.19.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankes W., Griesmacher A., Grünwald J., Schwartz-Albiez R., Keller R. A heparin-binding protein involved in inhibition of smooth-muscle cell proliferation. Biochem J. 1988 May 1;251(3):831–842. doi: 10.1042/bj2510831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert U. G., Flanagan S. G., Löffler S., Baczko K., ter Meulen V., Rima B. K. Antigenic determinants of measles virus hemagglutinin associated with neurovirulence. J Virol. 1994 Mar;68(3):1486–1493. doi: 10.1128/jvi.68.3.1486-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Sargiacomo M., Graeve L., Saltiel A. R., Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski M. K., Atkinson J. P. Membrane cofactor protein. Curr Top Microbiol Immunol. 1992;178:45–60. doi: 10.1007/978-3-642-77014-2_4. [DOI] [PubMed] [Google Scholar]
- Manchester M., Liszewski M. K., Atkinson J. P., Oldstone M. B. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Wild T. F., Rabourdin-Combe C., Gerlier D. A monoclonal antibody recognizes a human cell surface glycoprotein involved in measles virus binding. J Gen Virol. 1992 Oct;73(Pt 10):2617–2624. doi: 10.1099/0022-1317-73-10-2617. [DOI] [PubMed] [Google Scholar]
- Naniche D., Wild T. F., Rabourdin-Combe C., Gerlier D. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J Gen Virol. 1993 Jun;74(Pt 6):1073–1079. doi: 10.1099/0022-1317-74-6-1073. [DOI] [PubMed] [Google Scholar]
- Oglesby T. J., Allen C. J., Liszewski M. K., White D. J., Atkinson J. P. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med. 1992 Jun 1;175(6):1547–1551. doi: 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post T. W., Liszewski M. K., Adams E. M., Tedja I., Miller E. A., Atkinson J. P. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991 Jul 1;174(1):93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze B., Gross H. J., Brossmer R., Herrler G. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J Virol. 1991 Nov;65(11):6232–6237. doi: 10.1128/jvi.65.11.6232-6237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepley M. P., Racaniello V. R. A monoclonal antibody that blocks poliovirus attachment recognizes the lymphocyte homing receptor CD44. J Virol. 1994 Mar;68(3):1301–1308. doi: 10.1128/jvi.68.3.1301-1308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpuye O. A., Labarrere C. A., McIntyre J. A. Glycosylation of membrane cofactor protein (CD46) in human trophoblast, kidney and platelets. Biochim Biophys Acta. 1992 Jun 24;1121(3):301–308. doi: 10.1016/0167-4838(92)90161-6. [DOI] [PubMed] [Google Scholar]