Abstract
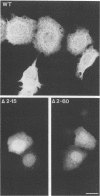
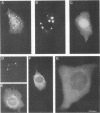
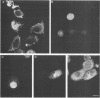
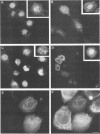
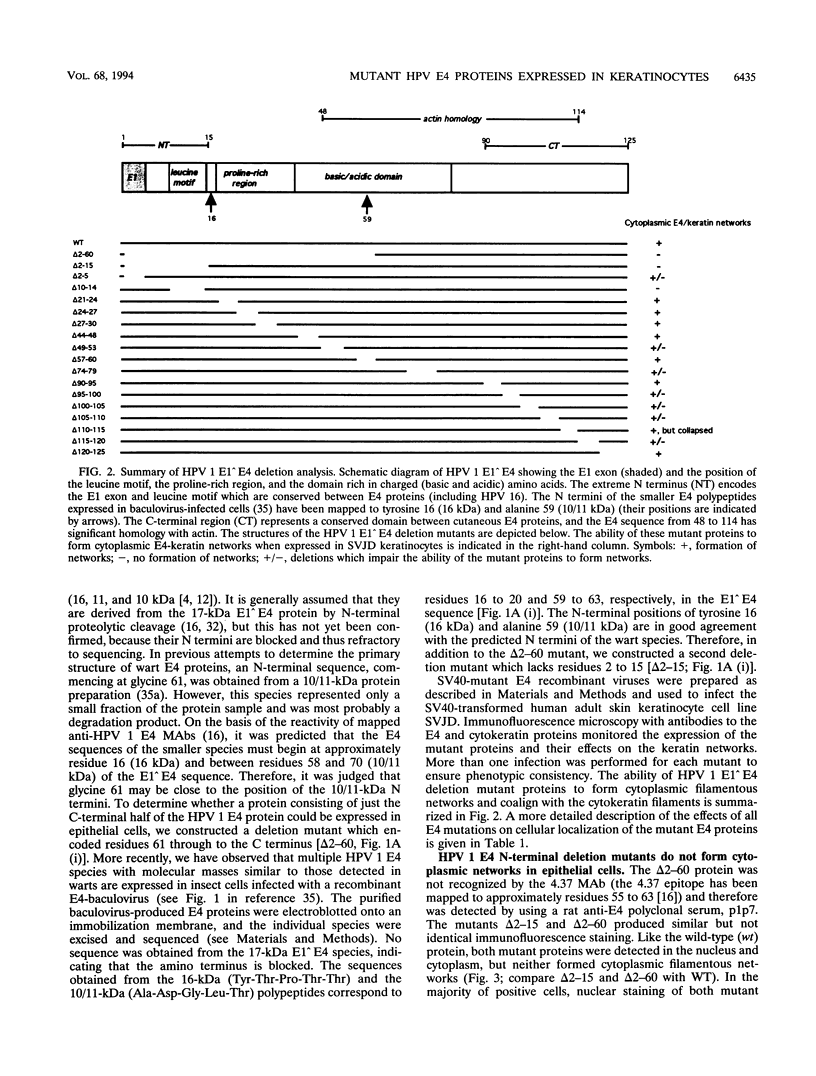
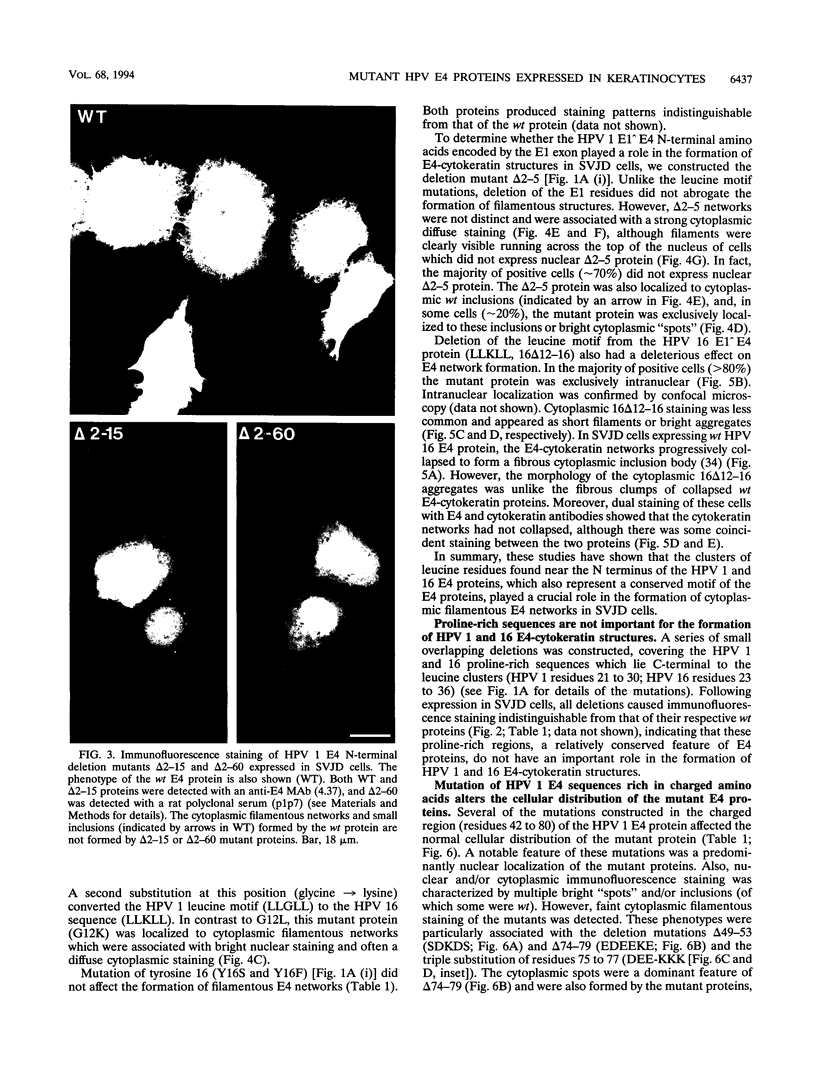
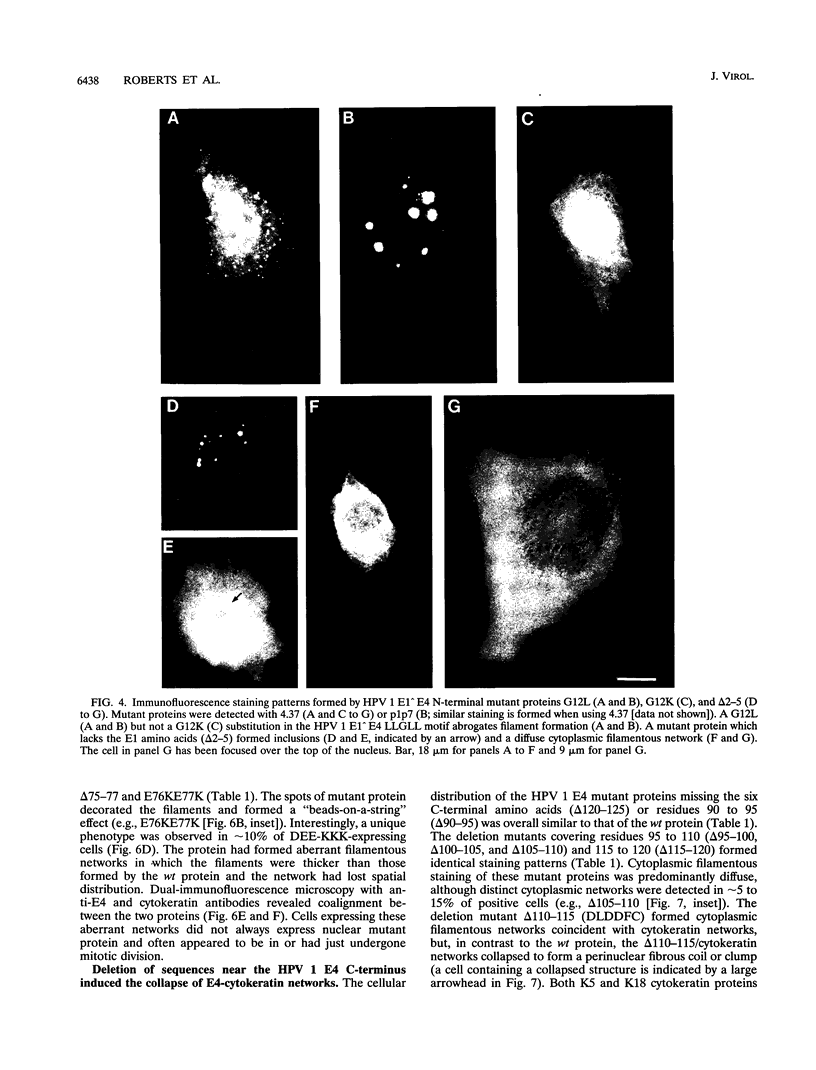
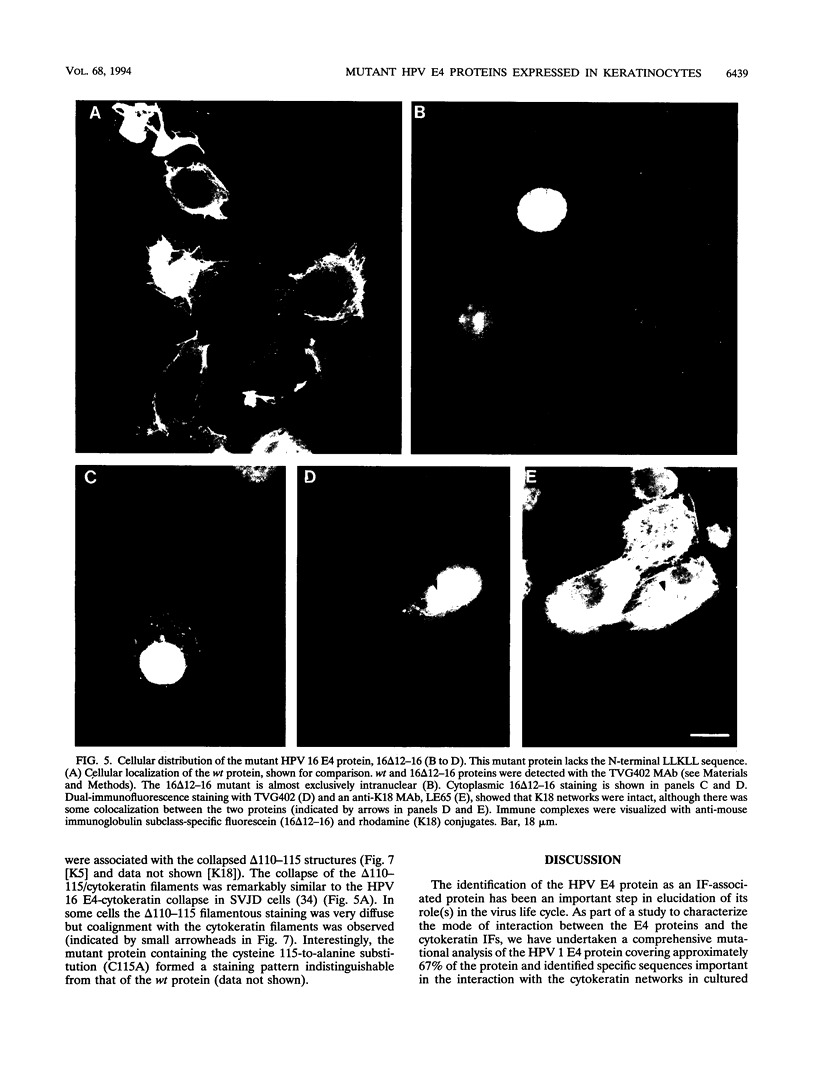
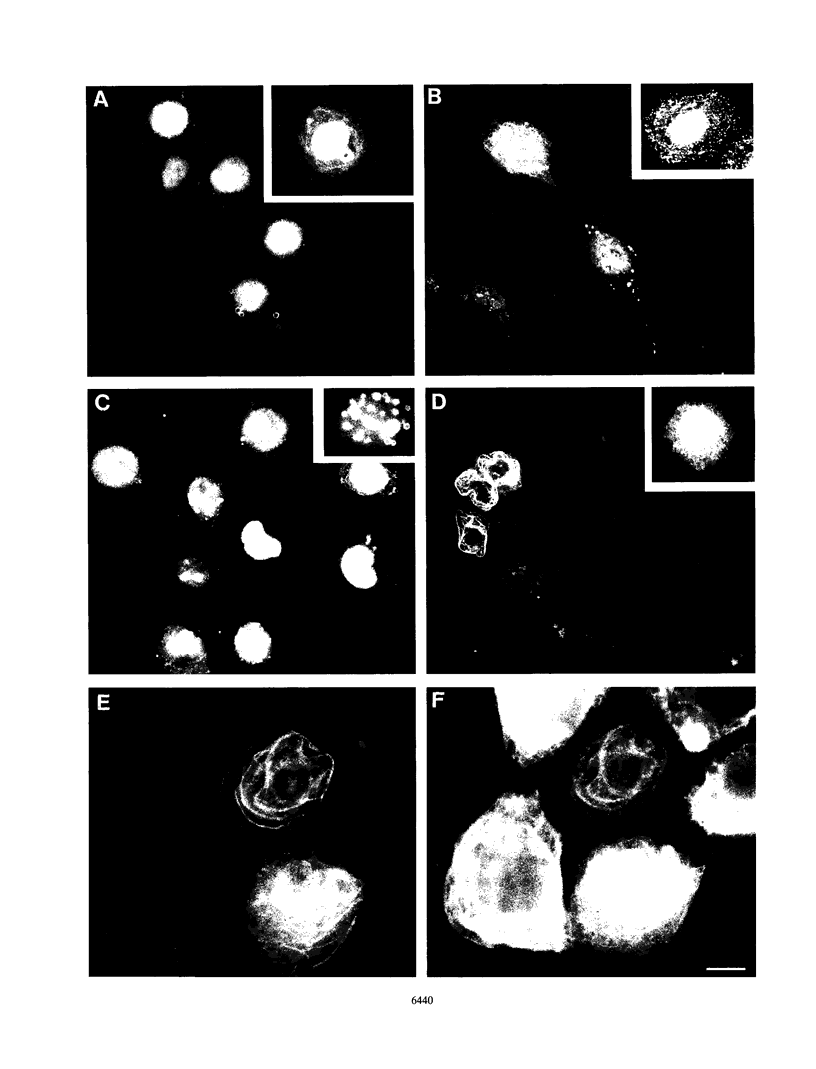
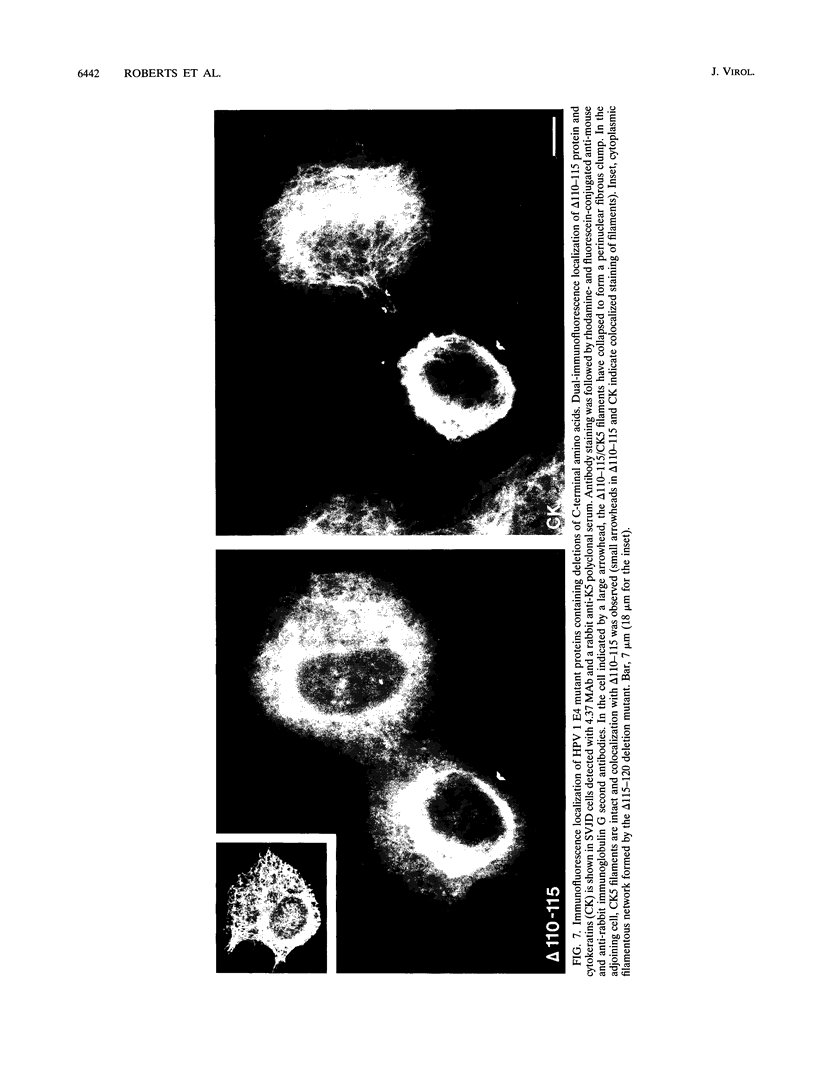
We have previously demonstrated that human papillomavirus type 1 (HPV 1) and 16 (HPV 16) E4 proteins form cytoplasmic filamentous networks which specifically colocalize with cytokeratin intermediate-filament (IF) networks when expressed in simian virus 40-transformed keratinocytes. The HPV 16 (but not the HPV 1) E4 protein induced the collapse of the cytokeratin networks. (S. Roberts, I. Ashmole, G. D. Johnson, J. W. Kreider, and P. H. Gallimore, Virology 197:176-187, 1993). The mode of interaction of E4 with the cytokeratin IFs is unknown. To identify E4 sequences important in mediating this interaction, we have constructed a large panel of mutant HPV (primarily HPV 1) E4 proteins and expressed them by using the same simian virus 40-epithelial expression system. Mutation of HPV 1 E4 residues 10 to 14 (LLGLL) abrogated the formation of cytoplasmic filamentous networks. This sequence corresponds to a conserved motif, LLXLL, found at the N terminus of other E4 proteins, and similar results were obtained on deletion of the HPV 16 motif, LLKLL (residues 12 to 16). Our findings indicate that this conserved motif is likely to play a central role in the association between E4 and the cytokeratins. An HPV 1 E4 mutant protein containing a deletion of residues 110 to 115 induced the collapse of the cytokeratin IFs in a manner analogous to the HPV 16 E4 protein. The sequence deleted, DLDDFC, is highly conserved between cutaneous E4 proteins. HPV 1 E4 residues 42 to 80, which are rich in charged amino acids, appeared to be important in the cytoplasmic localization of E4. In addition, we have mapped the N-terminal residues of HPV 1 E4 16-kDa and 10/11-kDa polypeptides expressed by using the baculovirus system and shown that they begin at tyrosine 16 and alanine 59, respectively. Similar-sized E4 proteins are also found in vivo. N-terminal deletion proteins, which closely resemble the 16-kDa and 10/11-kDa species, expressed in keratinocytes were both cytoplasmic and nuclear but did not form cytoplasmic filamentous networks. These findings support the postulate that N-terminal proteolytic processing of the E1-- E4 protein may modulate its function in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Howley P. M. Differential promoter utilization by the bovine papillomavirus in transformed cells and productively infected wart tissues. EMBO J. 1987 Apr;6(4):1027–1035. doi: 10.1002/j.1460-2075.1987.tb04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton G. J. Protein multiple sequence alignment and flexible pattern matching. Methods Enzymol. 1990;183:403–428. doi: 10.1016/0076-6879(90)83027-7. [DOI] [PubMed] [Google Scholar]
- Bhandari D. G., Levine B. A., Trayer I. P., Yeadon M. E. 1H-NMR study of mobility and conformational constraints within the proline-rich N-terminal of the LC1 alkali light chain of skeletal myosin. Correlation with similar segments in other protein systems. Eur J Biochem. 1986 Oct 15;160(2):349–356. doi: 10.1111/j.1432-1033.1986.tb09978.x. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Bryan J., Rodriguez M., Rose R. C., Strike D. G. Detection of human papillomavirus types 6 and 11 E4 gene products in condylomata acuminatum. J Med Virol. 1991 May;34(1):20–28. doi: 10.1002/jmv.1890340105. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Nasseri M., Wolinsky S. M., Broker T. R. Human papillomavirus types 6 and 11 mRNAs from genital condylomata acuminata. J Virol. 1987 Aug;61(8):2581–2588. doi: 10.1128/jvi.61.8.2581-2588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Reilly S. S., Broker T. R., Taichman L. B. Identification and mapping of human papillomavirus type 1 RNA transcripts recovered from plantar warts and infected epithelial cell cultures. J Virol. 1987 Jun;61(6):1913–1918. doi: 10.1128/jvi.61.6.1913-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Barber S., Symbula M., Snyder K., Saleh A. M., Roche J. K. Coexpression of the human papillomavirus type 16 E4 and L1 open reading frames in early cervical neoplasia. Virology. 1990 Sep;178(1):238–246. doi: 10.1016/0042-6822(90)90399-c. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C., Yaniv M. Production of spliced DNA copies of the cottontail rabbit papillomavirus genome in a retroviral vector. Ciba Found Symp. 1986;120:68–82. doi: 10.1002/9780470513309.ch6. [DOI] [PubMed] [Google Scholar]
- Doorbar J., Campbell D., Grand R. J., Gallimore P. H. Identification of the human papilloma virus-1a E4 gene products. EMBO J. 1986 Feb;5(2):355–362. doi: 10.1002/j.1460-2075.1986.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
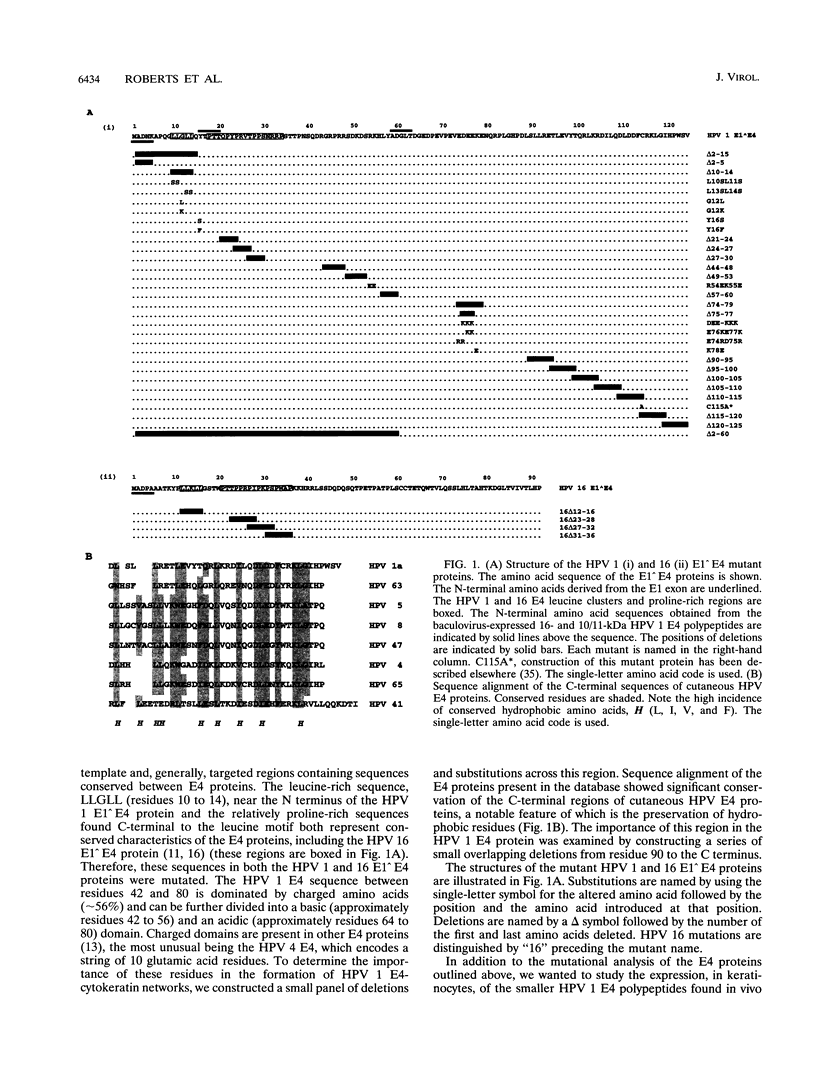
- Doorbar J., Coneron I., Gallimore P. H. Sequence divergence yet conserved physical characteristics among the E4 proteins of cutaneous human papillomaviruses. Virology. 1989 Sep;172(1):51–62. doi: 10.1016/0042-6822(89)90106-2. [DOI] [PubMed] [Google Scholar]
- Doorbar J., Ely S., Coleman N., Hibma M., Davies D. H., Crawford L. Epitope-mapped monoclonal antibodies against the HPV16E1--E4 protein. Virology. 1992 Mar;187(1):353–359. doi: 10.1016/0042-6822(92)90327-l. [DOI] [PubMed] [Google Scholar]
- Doorbar J., Ely S., Sterling J., McLean C., Crawford L. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature. 1991 Aug 29;352(6338):824–827. doi: 10.1038/352824a0. [DOI] [PubMed] [Google Scholar]
- Doorbar J., Evans H. S., Coneron I., Crawford L. V., Gallimore P. H. Analysis of HPV-1 E4 gene expression using epitope-defined antibodies. EMBO J. 1988 Mar;7(3):825–833. doi: 10.1002/j.1460-2075.1988.tb02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J., Parton A., Hartley K., Banks L., Crook T., Stanley M., Crawford L. Detection of novel splicing patterns in a HPV16-containing keratinocyte cell line. Virology. 1990 Sep;178(1):254–262. doi: 10.1016/0042-6822(90)90401-c. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990 Dec;111(6 Pt 2):2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Doorbar J., Smith K. J., Coneron I., Gallimore P. H. Phosphorylation of the human papillomavirus type 1 E4 proteins in vivo and in vitro. Virology. 1989 May;170(1):201–213. doi: 10.1016/0042-6822(89)90367-x. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Jareborg N., Burnett S. Immunofluorescent detection of bovine papillomavirus E4 antigen in the cytoplasm of cells permissive in vitro for viral DNA amplification. J Gen Virol. 1991 Sep;72(Pt 9):2269–2274. doi: 10.1099/0022-1317-72-9-2269. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison H. M., Grand R. J., Byrd P. J., Johnson G. D., Parton A., Gallimore P. H. The expression of the adenovirus 12 early region 1B 19K protein using a recombinant simian virus 40 system. J Gen Virol. 1990 Aug;71(Pt 8):1713–1722. doi: 10.1099/0022-1317-71-8-1713. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Hirochika R., Broker T. R., Chow L. T. A human papilloma virus type 11 transcript encoding an E1--E4 protein. Virology. 1987 Aug;159(2):433–439. doi: 10.1016/0042-6822(87)90482-x. [DOI] [PubMed] [Google Scholar]
- Neary K., Horwitz B. H., DiMaio D. Mutational analysis of open reading frame E4 of bovine papillomavirus type 1. J Virol. 1987 Apr;61(4):1248–1252. doi: 10.1128/jvi.61.4.1248-1252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Palefsky J. M., Winkler B., Rabanus J. P., Clark C., Chan S., Nizet V., Schoolnik G. K. Characterization of in vivo expression of the human papillomavirus type 16 E4 protein in cervical biopsy tissues. J Clin Invest. 1991 Jun;87(6):2132–2141. doi: 10.1172/JCI115245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo-Dilts D. A., Broker T. R., Chow L. T. Human papillomavirus type 1 produces redundant as well as polycistronic mRNAs in plantar warts. J Virol. 1990 Jun;64(6):3144–3149. doi: 10.1128/jvi.64.6.3144-3149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Roberts S., Ashmole I., Johnson G. D., Kreider J. W., Gallimore P. H. Cutaneous and mucosal human papillomavirus E4 proteins form intermediate filament-like structures in epithelial cells. Virology. 1993 Nov;197(1):176–187. doi: 10.1006/viro.1993.1578. [DOI] [PubMed] [Google Scholar]
- Roberts S., Ashmole I., Sheehan T. M., Davies A. H., Gallimore P. H. Human papillomavirus type 1 E4 protein is a zinc-binding protein. Virology. 1994 Aug 1;202(2):865–874. doi: 10.1006/viro.1994.1408. [DOI] [PubMed] [Google Scholar]
- Rogel-Gaillard C., Breitburd F., Orth G. Human papillomavirus type 1 E4 proteins differing by their N-terminal ends have distinct cellular localizations when transiently expressed in vitro. J Virol. 1992 Feb;66(2):816–823. doi: 10.1128/jvi.66.2.816-823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel-Gaillard C., Pehau-Arnaudet G., Breitburd F., Orth G. Cytopathic effect in human papillomavirus type 1-induced inclusion warts: in vitro analysis of the contribution of two forms of the viral E4 protein. J Invest Dermatol. 1993 Dec;101(6):843–851. doi: 10.1111/1523-1747.ep12371705. [DOI] [PubMed] [Google Scholar]
- Stamps A. C., Campo M. S. Mapping of two novel transcripts of bovine papillomavirus type 4. J Gen Virol. 1988 Dec;69(Pt 12):3033–3045. doi: 10.1099/0022-1317-69-12-3033. [DOI] [PubMed] [Google Scholar]
- Sterling J. C., Skepper J. N., Stanley M. A. Immunoelectron microscopical localization of human papillomavirus type 16 L1 and E4 proteins in cervical keratinocytes cultured in vivo. J Invest Dermatol. 1993 Feb;100(2):154–158. doi: 10.1111/1523-1747.ep12462790. [DOI] [PubMed] [Google Scholar]
- Stewart M. Intermediate filament structure and assembly. Curr Opin Cell Biol. 1993 Feb;5(1):3–11. doi: 10.1016/s0955-0674(05)80002-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y., Fuse A., Sekine H., Shirasawa H., Simizu B., Sugimoto M., Funahashi S. Human papillomavirus type 6 and 11 E4 gene products in condyloma acuminata. J Gen Virol. 1991 Mar;72(Pt 3):731–734. doi: 10.1099/0022-1317-72-3-731. [DOI] [PubMed] [Google Scholar]