Abstract
Germ-line mutations of the BRCA1 gene predispose women to early-onset breast and ovarian cancer by compromising the gene’s presumptive function as a tumor suppressor. Although the biochemical properties of BRCA1 polypeptides are not understood, their expression pattern and subcellular localization suggest a role in cell-cycle regulation. When resting cells are induced to proliferate, the steady-state levels of BRCA1 increase in late G1 and reach a maximum during S phase. Moreover, in S phase cells, BRCA1 polypeptides are hyperphosphorylated and accumulate into discrete subnuclear foci termed “BRCA1 nuclear dots.” BRCA1 associates in vivo with a structurally related protein termed BARD1. Here we show that the steady-state levels of BARD1, unlike those of BRCA1, remain relatively constant during cell cycle progression. However, immunostaining revealed that BARD1 resides within BRCA1 nuclear dots during S phase of the cell cycle, but not during the G1 phase. Nevertheless, BARD1 polypeptides are found exclusively in the nuclear fractions of both G1- and S-phase cells. Therefore, progression to S phase is accompanied by the aggregation of nuclear BARD1 polypeptides into BRCA1 nuclear dots. This cell cycle-dependent colocalization of BARD1 and BRCA1 indicates a role for BARD1 in BRCA1-mediated tumor suppression.
The BRCA1 tumor suppressor has been implicated in familial cases of early-onset breast and ovarian cancer (1, 2). However, the biochemical functions of its protein product are not defined and the mechanism by which it counters tumor formation during normal development is not understood. The major isoform of BRCA1 is a polypeptide of ≈220 kDa that bears several recognizable amino acid motifs: these include a zinc-binding RING domain that lies near the amino terminus, two nuclear localization signals, and two tandem copies of the BRCT motif that reside at the carboxyl terminus (2–5). BRCA1 associates in vivo with BARD1, a protein that also contains an amino-terminal RING domain and two carboxyl-terminal BRCT motifs (6). The interaction between these proteins is abolished by tumorigenic missense mutations in the RING domain of BRCA1, raising the possibility that tumor suppression is mediated by a heteromeric complex of BRCA1 and BARD1.
Products of the BRCA1 gene are found in a broad spectrum of cell and tissue types (2, 7, 8); however, the expression of this gene in most (9–12), but not all (13), cell types is tightly regulated during cell cycle progression. In resting cells, the levels of BRCA1 transcripts and polypeptides are either low or undetectable. However, after these cells receive a mitotic stimulus the steady-state levels of BRCA1 products rise in late G1, peak just prior to the onset of DNA synthesis, and persist for the duration of S phase and most of M phase. In addition, BRCA1 polypeptides become hyperphosphorylated as they begin to accumulate in late G1 (9). While not conclusive, these findings suggest that BRCA1 may be involved in some aspect of cell cycle regulation (9–12).
While there have been conflicting views about its subcellular distribution, recent studies indicate that BRCA1 resides predominately in the nuclei of normal cells (14–17). During S phase, when their levels are most abundant, BRCA1 polypeptides exist in distinct subnuclear bodies, termed “BRCA1 nuclear dots.” Although the function of these dots is not known, most, but not all, costain with antibodies that recognize HsRad51, a DNA-binding protein that shares extensive homology with the yeast Rad51 and Escherichia coli RecA proteins (18). HsRad51 promotes homologous pairing and single-strand exchange between DNA duplexes, and it has been implicated in a variety of nuclear processes, including DNA recombination, RNA transcription, and DNA repair (see ref. 18 for additional references). As such, the colocalization of BRCA1 and HsRad51 to the same subnuclear structures provides important clues about BRCA1 function (18).
To obtain additional insights into the function of BRCA1, we have examined the expression and subcellular distribution of BARD1 during cell cycle progression. In contrast to BRCA1, the steady-state levels of BARD1 remain relatively constant throughout the cell cycle. Subcellular fractionation of synchronized cell populations showed that BARD1 resides in the nuclei of proliferating cells, and two-color immunofluorescence with BARD1-specific antibodies revealed a punctate pattern of nuclear staining with nearly perfect colocalization of BARD1 and BRCA1. However, the punctate pattern of BARD1 immunostaining was observed in S-phase, but not in G1-phase, cells. Therefore, despite the presence of BARD1 polypeptides in the nucleus throughout cell cycle progression, their accumulation into BRCA1 nuclear dots is an S-phase-specific phenomenon that may require recruitment by BRCA1.
MATERIALS AND METHODS
Cells, Plasmids, and Antibodies.
The HBL-100 and T24 cell lines were obtained from the American Type Culture Collection, and normal human mammary epithelial cells (HMECs) were purchased from Clonetics (San Diego). Three different BARD1-specific antibody reagents were used in this study: a mouse polyclonal antiserum, a mouse monoclonal antibody, and an affinity-purified rabbit polyclonal antiserum. To prepare the latter, a cDNA fragment of human BARD1 was inserted into the BamHI/HindIII sites of the pMAL-c2 bacterial expression vector (New England Biolabs); the resultant plasmid encodes MBP-EE, a hybrid polypeptide composed of the E. coli maltose-binding protein (MBP) fused to residues 141–388 of BARD1. MBP-EE polypeptides were then purified from E. coli lysates by affinity chromatography on an amylose column (New England Biolabs) and conjugated to CNBr-activated Sepharose 4B (Pharmacia Biotech). The rabbit polyclonal antiserum raised against GST-EE, a hybrid polypeptide containing silkworm glutathione S-transferase fused to residues 141–388 of BARD1 (6), was then purified by sequential affinity chromatography on HiTrap protein A-Sepharose (Pharmacia Biotech) and MBP-EE-conjugated Sepharose 4B. The BARD1-specific mouse polyclonal antiserum and monoclonal antibody were raised by immunizing mice with the GST-EE polypeptide, as described elsewhere (M.-C.W.Y. and R.B., unpublished work). The monoclonal antibody was used for BARD1 immunoblots (e.g., Figs. 1 and 5). Monoclonal antibodies that recognize BRCA1 (MS110), cyclin A (Ab-3), NuMA (Ab-1), and α-tubulin (Ab-1) were purchased from Oncogene Research Products. The CDK2-specific antiserum (M2) was obtained from Santa Cruz Biotechnology.
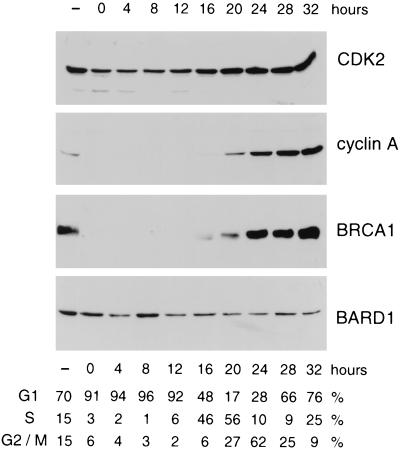
Figure 1.
Expression of BARD1 polypeptides during cell cycle progression. Contact-inhibited T24 cells were released from growth arrest by replating at low density. Synchronized cell populations were then lysed at the indicated time points, and equivalent aliquots of each lysate were subjected to Western analyses with antibodies specific for CDK2, cyclin A, BRCA1, and BARD1. The cell cycle distribution of the synchronized cultures at each time point was estimated by FACS analysis, as tabulated beneath the immunoblots.
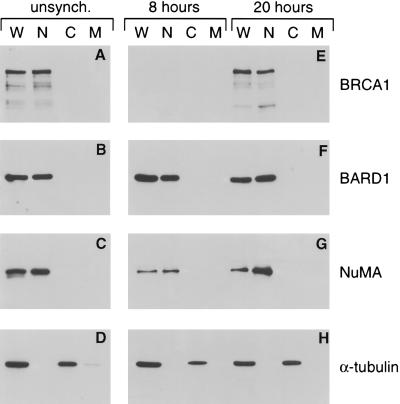
Figure 5.
BARD1 polypeptides reside within the nuclear compartment of G1- and S-phase cells. Whole cell (W), nuclear (N), cytoplasmic (C), and membrane (M) fractions were prepared from unsynchronized T24 cells (A–D), as well as from contact-inhibited cells that had been released from growth arrest for either 8 hr or 20 hr (E–H). Equivalent aliquots of each fraction were then subjected to Western analyses with antibodies specific for BRCA1 (A and E), BARD1 (B and F), nuclear matrix protein NuMA (C and G), and α-tubulin (D and H).
Cell Cycle Synchronization.
T24 cells were arrested in G0 by contact inhibition in 175-cm2 flasks. After at least 3 days of confluence, the cells were split 1:10 by seeding multiple 100-mm dishes at a concentration of ≈106 cells per dish. Ten dishes were harvested at each time point after replating—two for fluorescence-activated cell sorting (FACS) analysis and eight for Western analyses. To determine the cell cycle distribution at each time point, the contents of each dish were incubated for 10 min at room temperature in 2 ml of trypsin/EDTA solution [0.25% trypsin/0.1% EDTA in Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+; Mediatech]. The trypsinized cells were then washed in 10 ml of growth medium (McCoy’s 5A, 10% FBS) and resuspended in 1.5 ml of ice-cold PBS (without Ca2+ and Mg2+). After 3.5 ml of ice-cold 100% ethanol had been added dropwise, the cells were fixed at 4°C for at least 16 hr. The fixed cells were pelleted, resuspended in 1 ml of PI staining solution (50 μg/ml propidium iodide, 100 units/ml RNase A, 0.1% glucose in PBS without Ca2+ and Mg2+), incubated for at least 1 hr at room temperature, and analyzed on a FACScan flow cytometer (Becton Dickinson). For Western analyses the contents of eight dishes were lysed in a total of 300 μl of RIPA buffer (50 mM Tris⋅HCl, pH 7.6/150 mM NaCl/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS) containing complete protease inhibitor mixture (Boehringer Mannheim) and phosphatase inhibitors (5 mM β-glycerophosphate, 10 mM benzamidine, and 0.5 mM sodium orthovanadate). The lysate was swirled in a Vortex mixer for 10 min at 4°C and cleared of insoluble debris by centrifugation for 10 min at 12,000 rpm in a Microfuge at 4°C. The protein concentration of each supernatant was determined by using the BCA Protein Assay Reagent (Pierce). Western analyses were conducted by enhanced chemiluminescence (ECL; Amersham) using 80 μg of lysate for BRCA1 immunoblots and 30 μg for CDK2, cyclin A, and BARD1 immunoblots.
Immunofluorescence Microscopy.
Approximately 2.5 × 106 cells were seeded onto microscope slides in a 150-mm culture dish. After 2 days, the cells were fixed with 4% paraformaldehyde for 15 min and permeabilized in 0.2% Triton X-100 for 10 min. Nonspecific staining was blocked by a 60-min incubation with 2% BSA in PBS (BSA/PBS solution) and two 15-min treatments with the Avidin/Biotin Blocking Kit (Vector Laboratories). After a 60-min incubation with primary antibody, the cells were treated with 8 μg/ml biotinylated secondary antibody (Vector Laboratories) for 45 min and 20 μg/ml fluorescein-labeled avidin D (Vector Laboratories) for an additional 30 min. The cells were then treated with 100 μg/ml RNase A in PBS for 20 min at 37°C, and with 10 μg/ml propidium iodide in PBS for an additional 20 min. The stained cells were mounted under coverslips with Vectashield mounting medium (Vector Laboratories) and sealed with nail polish. Immunofluorescence was recorded using a confocal microscope equipped with a MRC-1024 Lasersharp confocal imaging system (Bio-Rad). All the above procedures were performed at room temperature except where indicated. For colocalization experiments, the cells were incubated simultaneously with two primary antibodies from different species (rabbit and mouse) for 60 min. After treatment with Texas red-conjugated anti-rabbit goat IgG (Vector Laboratories) and biotinylated anti-mouse goat IgG (Vector Laboratories) for 45 min, the cells were incubated for an additional 30 min with fluorescein-avidin D. The immunostained cells were then mounted as described above (without RNase A digestion and propidium iodide staining). For experiments presented in Fig. 3, a 10-μg aliquot of the BARD1- or BRCA1-specific monoclonal antibody was preabsorbed by overnight incubation at 4°C with 50 μg of either the parental GST polypeptide or the cognate immunogen (GST-EE or GST-BR304, respectively) immobilized on glutathione-agarose beads.
Figure 3.
Cell cycle specificity of BARD1 immunostaining. Synchronized populations of T24 cells were costained with propidium iodide and either the BRCA1-specific MS110 monoclonal antibody (A–D) or the BARD1-specific monoclonal antibody (E–H). The composite images of two-color staining are illustrated. The cells were harvested at either 8 hr (A and E) or 20 hr (B–D and F–H) after synchronous release from growth arrest. In some experiments the monoclonal antibodies were preabsorbed with an excess of the BRCA1 immunogen (GST-BR304; C), the BARD1 immunogen (GST-EE; G) or the parental GST polypeptide (D and H).
Cell Fractionation.
To prepare whole cell lysates of unsynchronized T24 cells, the cellular contents of two 150-mm dishes (≈1.7 × 107 cells per dish) were lysed in 1 ml of RIPA buffer (containing protease and phosphatase inhibitors, as described above). Whole cell lysates of synchronized T24 cells (8 hr or 20 hr after replating) were prepared by lysing the contents of six 150-mm dishes in 1 ml of RIPA buffer (≈2.6 × 106 cells per dish). Each whole cell lysate was swirled in a Vortex mixer for 15 min at 4°C and cleared of insoluble debris by centrifugation for 10 min at 12,000 rpm in a Microfuge at 4°C. To prepare membrane, cytoplasmic, and nuclear fractions from unsynchronized cells, the contents of seven 150-mm dishes (≈1.7 × 107 cells per dish) were resuspended in 5 ml of hypotonic lysis buffer and processed as described by Abrams et al. (19). For synchronized cells, the contents of 25 150-mm dishes (≈2.6 × 106 cells per dish) were resuspended in 5 ml of hypotonic lysis buffer and processed to prepare subcellular fractions (19). For detection of BRCA1, equivalent volumes of each fraction (corresponding to 300 μg of whole cell lysate) were immunoprecipitated with the BRCA1-specific rabbit antiserum (6) and the immunoprecipitates were subjected to Western analysis with the BRCA1-specific MS110 monoclonal antibody. For detection of BARD1, NuMA, and α-tubulin, equivalent volumes of each fraction (corresponding to 10 μg of whole cell lysate) were directly evaluated by Western analysis with the appropriate monoclonal antibody.
RESULTS
The Steady-State Levels of BARD1 Remain Constant During Cell Cycle Progression.
To compare the expression of BARD1 and BRCA1 polypeptides with respect to the cell cycle, we measured their steady-state levels in synchronized populations of T24 bladder carcinoma cells. Parallel cultures of T24 cells were arrested in G0 by contact inhibition and released from cell cycle arrest by replating at low density, and individual cultures were harvested at various times after replating (9). The cell cycle distribution profile of each culture was then determined by FACS analysis and protein levels were evaluated by immunoblotting. As shown in Fig. 1, these cells display the expected expression patterns for known cell cycle regulatory molecules. For example, CDK2 is present throughout the cell cycle and its steady-state levels increase modestly in S and G2/M cells. However, the levels of its regulatory subunit, cyclin A, rise dramatically after the G1/S transition. BRCA1 shows an expression profile similar to that described in a previous study of T24 cells (9); thus, while few, if any, BRCA1 products are detected in resting or G1 cells, BRCA1 expression increases markedly as cells enter S phase. In contrast, comparable levels of BARD1 polypeptides are seen at all time points, indicating that BARD1 expression remains relatively constant throughout the cell cycle (Fig. 1).
BARD1 Polypeptides Reside in Discrete Subnuclear Bodies.
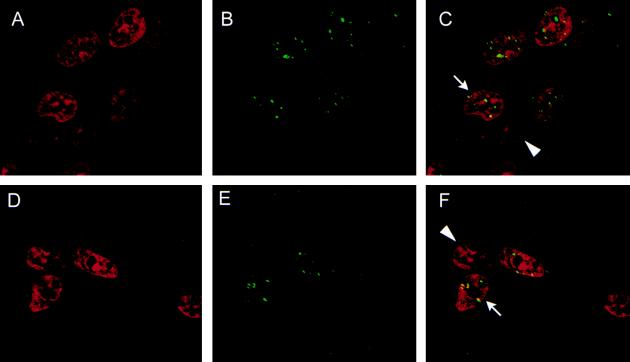
The subcellular distribution of BARD1 polypeptides was evaluated by immunofluorescent staining of unsynchronized HBL-100 cells, a human line of normal mammary epithelial cells that was presumably immortalized by transforming sequences of the simian virus 40 papovavirus (20). For this purpose we prepared a mouse polyclonal antiserum against residues 141–388 of BARD1, a segment that bears no homology to other known proteins (6). After staining with either the BARD1-specific antiserum or a BRCA1-specific monoclonal antibody (MS110; Oncogene Research Products) (15), the cells were counterstained with propidium iodide to highlight the nuclei. As shown in Fig. 2, a characteristic pattern of BRCA1 subcellular distribution was observed in which BRCA1 nuclear dots appeared in some, but not all, interphase cells (Fig. 2 A–C). Likewise, the BARD1-specific antiserum generated a similar pattern of punctate nuclear staining in a subset of interphase cells (Fig. 2 D–F). The same results were also obtained when T24 colon carcinoma cells and primary HMECs were used (data not shown).
Figure 2.
A punctate pattern of nuclear staining observed with BRCA1- and BARD1-specific antibodies. Asynchronous HBL-100 cells were stained with propidium iodide to highlight the nucleus (red, A and D) and with either the BRCA1-specific MS110 monoclonal antibody (green, B) or a BARD1-specific mouse polyclonal antiserum (green, E). C is a composite image of A and B, and F is a composite of D and E; in the composite images yellow is produced where the red and green signals overlap. Punctate staining of BRCA1 and BARD1 is observed in some (arrows) but not all (arrowheads) interphase nuclei (C and F).
BARD1-Containing Nuclear Foci Appear Specifically in S-Phase Cells.
Scully et al. (18) have shown that the nuclear dot pattern of BRCA1 staining arises specifically during S phase of the cell cycle. To determine whether the subnuclear structures that stain with BARD1 have a similar cell cycle dependence, synchronized populations of T24 cells were stained with BARD1- or BRCA1-specific monoclonal antibodies. In particular, we analyzed cells harvested at 8 hr (91% G1-phase cells) and 20 hr (56% S-phase cells) after replating. As shown in Fig. 3, cells bearing BRCA1 nuclear dots were abundant in the S-phase population (Fig. 3B) but were rarely observed in the G1 population (Fig. 3A). The specificity of BRCA1 staining was confirmed in blocking experiments in which the primary antibody was preabsorbed with a matrix-bound protein containing silkworm GST fused to the amino-terminal 304 residues of BRCA1—the same BRCA1 moiety used to generate the MS110 monoclonal antibody (15). As expected, staining of BRCA1 nuclear dots was abolished by preabsorption with the GST-BRCA1 fusion protein (Fig. 3C) but not with the parental GST polypeptide (Fig. 3D). Immunofluorescence analysis of synchronized cell populations revealed that the appearance of BARD1-staining foci with respect to the cell cycle resembles that of BRCA1 nuclear dots. Thus, these foci are present in most cells of the S-phase population (Fig. 3F) but not the G1 population (Fig. 3E). Moreover, the staining of S-phase cells with BARD1-specific antibodies was ablated by preabsorption with GST-EE, a polypeptide containing GST fused to residues 141–388 of BARD1 (Fig. 3G) but not by GST itself (Fig. 3H). These data show that the BARD1-staining nuclear structure arises in an S-phase-specific fashion reminiscent of the BRCA1 nuclear dots.
BARD1 and BRCA1 Polypeptides Colocalize in BRCA1 Nuclear Dots.
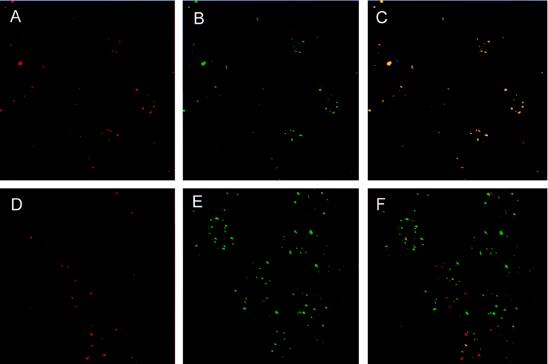
If BARD1 is a physiologically relevant partner of BRCA1, then the two proteins should reside in the same subcellular structures. Therefore, to determine whether the S-phase nuclear foci recognized by BARD1- and BRCA1-specific antibodies are one and the same, two-color immunofluorescence studies were conducted by staining HBL-100 cells simultaneously with an affinity-purified BARD1-specific rabbit antiserum and a mouse monoclonal antibody that recognizes either BRCA1 or PML; the latter is a RING protein that resides in distinct subnuclear structures referred to as PML oncogenic domains (PODs) (21–23). Images of BARD1-staining (red) and BRCA1- or PML-staining (green) from the same cells were then collected both separately and conjointly. As illustrated in Fig. 4, BARD1 staining coincides almost perfectly with the BRCA1 nuclear dots of HBL-100 cells (Fig. 4 A–C). In contrast, BARD1-staining structures are distributed randomly with respect to the PML-oncogenic domains (Fig. 4. D–F). Similar results were obtained in two-color immunofluorescence studies of HMECs (data not shown). These data demonstrate that BARD1 specifically colocalizes with BRCA1 in the same subnuclear bodies.
Figure 4.
BARD1 is a component of the BRCA1 nuclear dots. Asynchronous HBL-100 cells were costained with an affinity-purified BARD1-specific rabbit antiserum (red, A and D) and either a BRCA1-specific (green, B) or PML-specific (green, E) monoclonal antibody. The composite images of two-color staining demonstrate that BARD1 colocalizes with BRCA1 (C) but not with PML (F).
Subcellular Distribution of BARD1 Polypeptides During Cell Cycle Progression.
The BARD1-staining nuclear foci are apparent by immunofluorescence microscopy only after the onset of S phase (Fig. 3). Nevertheless, Western analysis of lysates from synchronized cell populations shows that the steady-state levels of BARD1 polypeptides remain relatively constant throughout the cell cycle (Fig. 1). Where, then, does BARD1 reside in G1-phase cells? To address this question, the subcellular distribution of BARD1 polypeptides was initially examined by Western analyses of nuclear, cytoplasmic, and membrane fractions prepared from asynchronous T24 cells. As shown in Fig. 5, BARD1 and BRCA1 were concentrated in the nuclear fraction along with the nuclear matrix protein NuMA (24) (Fig. 5A–C). In contrast, α-tubulin was found exclusively in the cytoplasmic and membrane compartments (Fig. 5D), indicating that there was little, if any, cross-contamination of the nuclear compartment with cytosolic proteins. Identical results were obtained by Western analysis of subcellular fractions from synchronized populations of T24 cells harvested at 8 hr (98% G1 cells) and 20 hr (52% S-phase cells) after release from cell cycle arrest (Fig. 5 E–H). Hence, during the G1 phase of the cell cycle, when BARD1 is not found in BRCA1 nuclear dots by immunofluorescent staining, the analysis of subcellular fractions reveals it to be predominantly a nuclear protein.
DISCUSSION
In most cell types BRCA1 expression remains low or undetectable until just prior to the G1/S transition (9–12). As S phase begins, the expression of BRCA1 increases dramatically and its protein products aggregate into nuclear dots (18). However, the nature and composition of these subnuclear domains remains unclear. Are they preexisting structures into which BRCA1 accumulates as cells enter S phase or do they represent newly assembled organelles that arise de novo with the onset of S phase? The mechanism underlying the accumulation of BRCA1 polypeptides into nuclear dots is equally unclear, although it may be significant that BRCA1 also undergoes hyperphosphorylation at this stage of the cell cycle (9).
Our previous studies had shown that BARD1 and BRCA1 associate with one another in vivo and that both proteins bear a similar arrangement of protein motifs, including an amino-terminal RING domain and two carboxyl-terminal BRCT domains (6). Since tumor-associated missense mutations of BRCA1 disrupt its in vivo interaction with BARD1, the formation of BRCA1/BARD1 heterodimers is likely to be a critical event in BRCA1-mediated tumor suppression. The present results indicate that BARD1, unlike BRCA1, is expressed at comparable levels throughout the cell cycle. In addition, Western analyses of subcellular fractions from synchronized cell populations demonstrate that BARD1 remains in the nuclear compartment of G1- and S-phase proliferating cells. Moreover, immunofluorescence microscopy reveals a punctate distribution of BARD1 polypeptides similar to that of BRCA1 nuclear dots, and two-color immunostaining showed that BARD1 and BRCA1 colocalize almost perfectly in the nuclei of these cells. These results, together with our previous data (6), provide compelling evidence that the in vivo association of BRCA1 and BARD1 is physiologically relevant. The same pattern of immunostaining with BRCA1- and BARD1-specific antibodies was observed in bladder carcinoma cells (T24), immortalized cells from normal mammary epithelium (HBL-100), as well as primary HMECs; thus, the colocalization of BRCA1 and BARD1 in nuclear dots appears to be independent of cell type and the degree of neoplastic transformation.
To our surprise, immunostaining with BARD1-specific antibodies was not observed in G1 cells, despite the fact that BARD1 polypeptides were readily detected by Western analysis of nuclear fractions derived from these cells. Several explanations can be invoked to account for this phenomenon. For example, the epitopes recognized by the BARD1-specific antibodies may be masked during certain stages of the cell cycle by interactions with other macromolecules. However, identical results were obtained with three different reagents raised against a substantial segment of human BARD1 (residues 141–388): an affinity-purified rabbit polyclonal antiserum, a mouse polyclonal antiserum, and a mouse monoclonal antibody. Furthermore, attempts to unmask hidden epitopes with heat or high salt failed to elicit BARD1-specific staining in G1 cells, despite the fact that the monoclonal antibody readily detects denatured BARD1 polypeptides (unpublished data). Thus, a more plausible explanation for this phenomenon is that BARD1 polypeptides are distributed diffusely within the nuclei of G1 cells at concentrations too low for immunodetection. In contrast, the S-phase-dependent accumulation of BARD1 into BRCA1 dots presumably increases their local concentration to levels detectable by immunofluorescence microscopy.
If BARD1 polypeptides are diffusely distributed in the nuclei of G1 cells, then all, or at least a significant subset, of these polypeptides must be recruited into the BRCA1 nuclear dots as cells progress into S phase. The present data do not address whether this relocalization of BARD1 can occur independently of BRCA1 or whether BARD1 accumulation into the dots requires the prior formation of BRCA1/BARD1 heterodimers. In this regard it would be interesting to see the nuclear distribution of BARD1 in cells that lack functional BRCA1; this should be feasible once cell lines are established from either Brca1-null mice or breast carcinomas of BRCA1 mutation carriers.
Germ-line mutations of either BRCA1 or BRCA2 are responsible for most cases of familial breast cancer. Thus, it is intriguing to note that these genes share a number of other similarities. First, unlike most tumor suppressors, BRCA1 and BRCA2 are rarely mutated in truly sporadic cases of breast cancer (25–28). Second, the phylogenetic conservation of both genes is remarkably poor—for example, the mouse and human orthologs of their protein products exhibit only 58% identity at the amino acid level (7, 29–33). Third, the transcription of both genes is coordinately induced by estrogen (34). Fourth, the expression patterns of BRCA1 and BRCA2 with respect to the cell cycle are almost indistinguishable: both are induced in late G1 upon mitogenic stimulation of quiescent cells, and the levels of their gene products peak just prior to DNA synthesis (9–12, 35, 36). These intriguing parallels were underscored recently by the discovery that BRCA2 also interacts in vivo with HsRad51 (33, 37). Although the subcellular localization of BRCA2 has not yet been described, these findings suggest that BRCA1 and BRCA2 normally serve as components of a common biochemical pathway involving the HsRad51 protein (18, 33, 37). As such, the disruption of this pathway by mutations in BRCA1 or BRCA2 may be a critical step in the development of hereditary breast cancer. The specific localization of BARD1 into the BRCA1 nuclear dots of S-phase cells suggests that it too may be an essential component of a HsRad51-associated pathway of tumor suppression.
Acknowledgments
We thank Ross Payne, Roger Schultz, and Gerd Maul for their invaluable advice on immunofluorescence microscopy. We are also especially grateful to the Neuroscience Research Center at University of Texas Southwestern for kindly providing access to their MRC-1024 confocal microscope. This work was supported by grants CA61011 (R.B.) and CA60650 (A.M.B.) from the National Cancer Institute. R.B. is the recipient of a Faculty Research Award from the American Cancer Society (FRA-421). Y.J. was supported in part by the Leukemia Association of North Central Texas and National Cancer Institute Cancer Immunology Training Grant T32-CA09082.
ABBREVIATIONS
- FACS
fluorescence-activated cell sorting
- GST
glutathione S-transferase
- HMEC
normal human mammary epithelial cell
References
- 1.Hall J M, Lee M K, Newman B, Morrow J E, Anderson L A, Huey B, King M-C. Science. 1990;250:1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 2.Miki Y, Swensen J, Shattuck-Eidens D, Futreal P A, Harshman K, et al. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 3.Chen C-f, Li S, Chen Y, Chen P-L, Sharp Z D, Lee W-H. J Biol Chem. 1996;271:32863–32868. doi: 10.1074/jbc.271.51.32863. [DOI] [PubMed] [Google Scholar]
- 4.Thakur S, Zhang H B, Peng Y, Le H, Carroll B, Ward T, Yao J, Farid L M, Couch F J, Wilson R B, Weber B L. Mol Cell Biol. 1997;17:444–452. doi: 10.1128/mcb.17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koonin E V, Altschul S F, Bork P. Nat Genet. 1996;13:266–267. doi: 10.1038/ng0796-266. [DOI] [PubMed] [Google Scholar]
- 6.Wu L C, Wang Z W, Tsan J T, Spillman M A, Phung A, Xu X L, Yang M-C, Hwang L-Y, Bowcock A M, Baer R. Nat Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 7.Lane T F, Deng C, Elson A, Lyu M S, Kozak C A, Leder P. Genes Dev. 1995;9:2712–2722. doi: 10.1101/gad.9.21.2712. [DOI] [PubMed] [Google Scholar]
- 8.Marquis S T, Rajan J V, Wynshaw-Boris A, Xu J, Yin G Y, Abel K J, Weber B L, Chodosh L A. Nat Genet. 1995;11:17–26. doi: 10.1038/ng0995-17. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Farmer A A, Chen C-F, Jones D C, Chen P-L, Lee W-H. Cancer Res. 1996;56:3168–3172. [PubMed] [Google Scholar]
- 10.Vaughn J P, Davis P L, Jarboe M D, Huper G, Evans A C, Wiseman R W, Futreal P A, Marks J R. Cell Growth Differ. 1996;7:711–715. [PubMed] [Google Scholar]
- 11.Gudas J M, Li T, Nguyen H, Jensen D, Rauscher F J, III, Cowen K H. Cell Growth Differ. 1996;7:717–723. [PubMed] [Google Scholar]
- 12.Rajan J V, Wang M, Marquis S T, Chodosh L A. Proc Natl Acad Sci USA. 1996;93:13078–13083. doi: 10.1073/pnas.93.23.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aprelikova O, Kuthiala A, Bessho M, Etheir S, Liu E T. Oncogene. 1996;13:2487–2491. [PubMed] [Google Scholar]
- 14.Chen Y, Chen C-F, Riley D J, Allred D C, Chen P-L, Hoff D V, Osborne C K, Lee W-H. Science. 1995;270:789–791. doi: 10.1126/science.270.5237.789. [DOI] [PubMed] [Google Scholar]
- 15.Scully R, Ganesan S, Brown M, Caprio J A D, Cannistra S A, Feunteun J, Schnitt S, Livingston D M. Science. 1996;272:123–125. doi: 10.1126/science.272.5258.123. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Chen P-L, Riley D J, Lee W-H, Allred D C, Osborne C K. Science. 1996;272:125–126. doi: 10.1126/science.272.5258.125. [DOI] [PubMed] [Google Scholar]
- 17.Thomas J E, Smith M, Rubinfeld B, Gutowski M, Beckmann R P, Polakis P. J Biol Chem. 1996;271:28630–28635. doi: 10.1074/jbc.271.45.28630. [DOI] [PubMed] [Google Scholar]
- 18.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston D M. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 19.Abrams H D, Rohrschneider L R, Eisenman R N. Cell. 1982;29:427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- 20.Caron de Fromentel C, Nardeux P C, Soussi T, Lavialle C, Estrade S, Carloni G, Chandrasekaran K, Cassingena R. Exp Cell Res. 1985;160:83–94. doi: 10.1016/0014-4827(85)90238-1. [DOI] [PubMed] [Google Scholar]
- 21.Dyck J A, Maul G G, Wilson H, Miller J, Chen J D, Kakizuka A, Evans R M. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 22.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 23.Koken M H M, Puvion-Dutilleul F, Guillemin M C, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, Degos L, Puvion E, de The H. EMBO J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyderson B K, Pettijohn D E. Cell. 1980;22:489–499. doi: 10.1016/0092-8674(80)90359-1. [DOI] [PubMed] [Google Scholar]
- 25.Futreal P A, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, et al. Science. 1994;266:120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- 26.Lancaster J M, Wooster R, Mangion J, Phelan C M, Cochran C, et al. Nat Genet. 1996;13:238–240. doi: 10.1038/ng0696-238. [DOI] [PubMed] [Google Scholar]
- 27.Teng D H-F, Bogden R, Mitchell J, Baumgard M, Bell R, Berry S, Davis T, Ha P C, Kehrer R, Jammulapati S, Chen Q, Offit K, Skolnick M H, Tavtigian S V, Jhanwar S, Swedlund B, Wong A K C, Kamb A. Nat Genet. 1996;13:241–244. doi: 10.1038/ng0696-241. [DOI] [PubMed] [Google Scholar]
- 28.Miki Y, Katagiri T, Kasumi F, Yoshimoto T, Nakamura Y. Nat Genet. 1996;13:245–247. doi: 10.1038/ng0696-245. [DOI] [PubMed] [Google Scholar]
- 29.Abel K J, Xy J, Yin G Y, Lyons R H, Meisler M H, Weber B L. Hum Mol Genet. 1995;4:2265–2273. doi: 10.1093/hmg/4.12.2265. [DOI] [PubMed] [Google Scholar]
- 30.Sharan S K, Wims M, Bradley A. Hum Mol Genet. 1995;4:2275–2278. doi: 10.1093/hmg/4.12.2275. [DOI] [PubMed] [Google Scholar]
- 31.Bennett M L, Haugen-Strano A, Cochran C, Brownlee H A, Fiedorek F T J, Wiseman R W. Genomics. 1995;29:576–581. doi: 10.1006/geno.1995.9963. [DOI] [PubMed] [Google Scholar]
- 32.Connor F, Smith A, Wooster R, Stratton M, Dixon A, Campbell E, Tait T-M, Freeman T, Ashworth A. Hum Mol Genet. 1997;6:291–300. doi: 10.1093/hmg/6.2.291. [DOI] [PubMed] [Google Scholar]
- 33.Sharan S K, Morimatsu M, Albrecht U, Lim D-S, Regel E, Dinh C, Sands A, Eichele G, Hasty P, Bradley A. Nature (London) 1997;386:804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- 34.Spillman M A, Bowcock A M. Oncogene. 1996;16:1639–1645. [PubMed] [Google Scholar]
- 35.Vaughn J P, Cirisano F D, Huper G, Berchuck A, Futreal P A, Marks J R, Iglehart J D. Cancer Res. 1996;56:4590–4594. [PubMed] [Google Scholar]
- 36.Wang S-C, Lin S-H, Su L-K, Hung M-C. Biochem Biophys Res Commun. 1997;234:247–251. doi: 10.1006/bbrc.1997.6544. [DOI] [PubMed] [Google Scholar]
- 37.Mizuta R, LaSalle J M, Cheng H-L, Shinohara A, Ogawa H, Copeland N, Jenkins N A, Lalande M, Alt F W. Proc Natl Acad Sci USA. 1997;94:6927–6932. doi: 10.1073/pnas.94.13.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]