Abstract
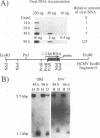
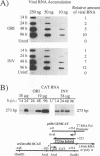
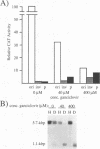
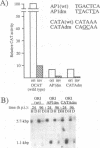
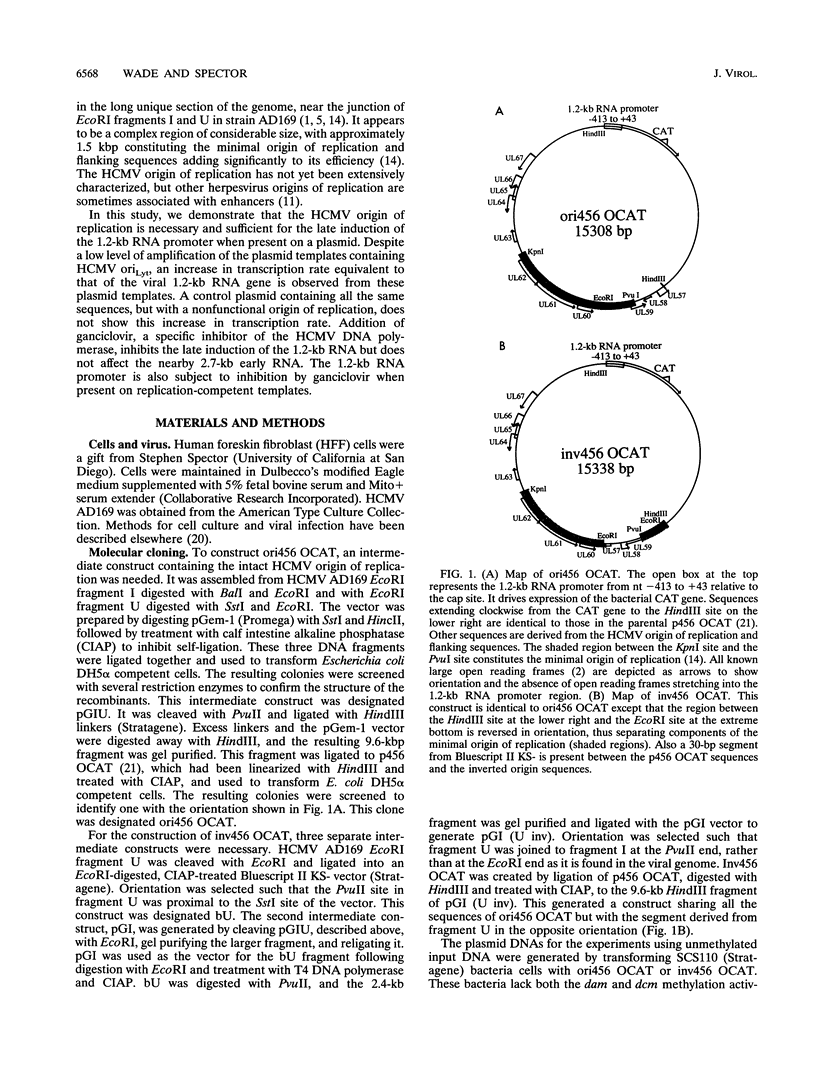
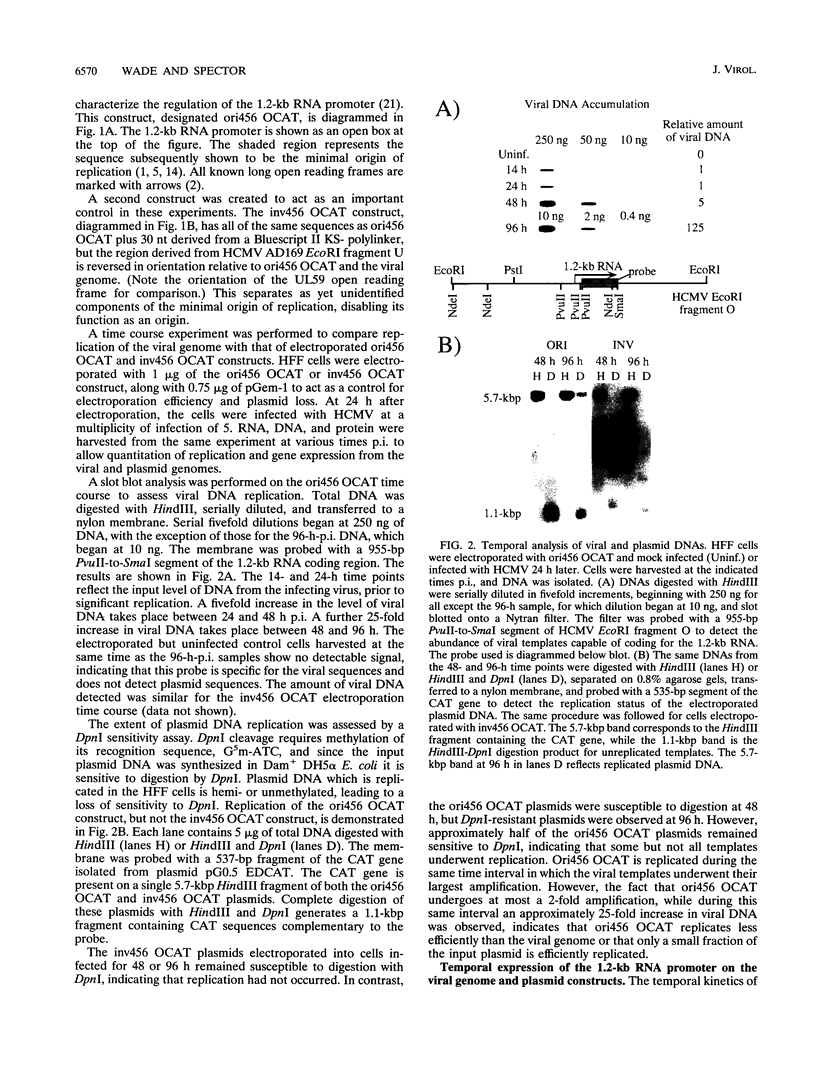
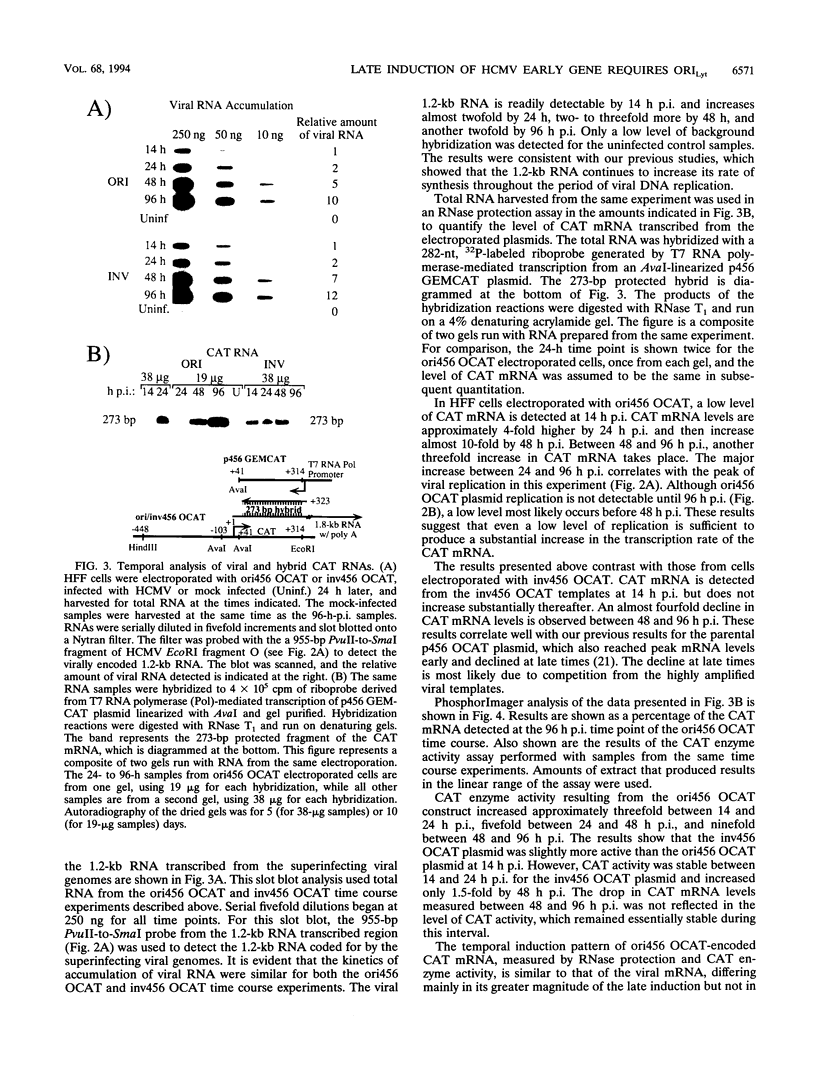
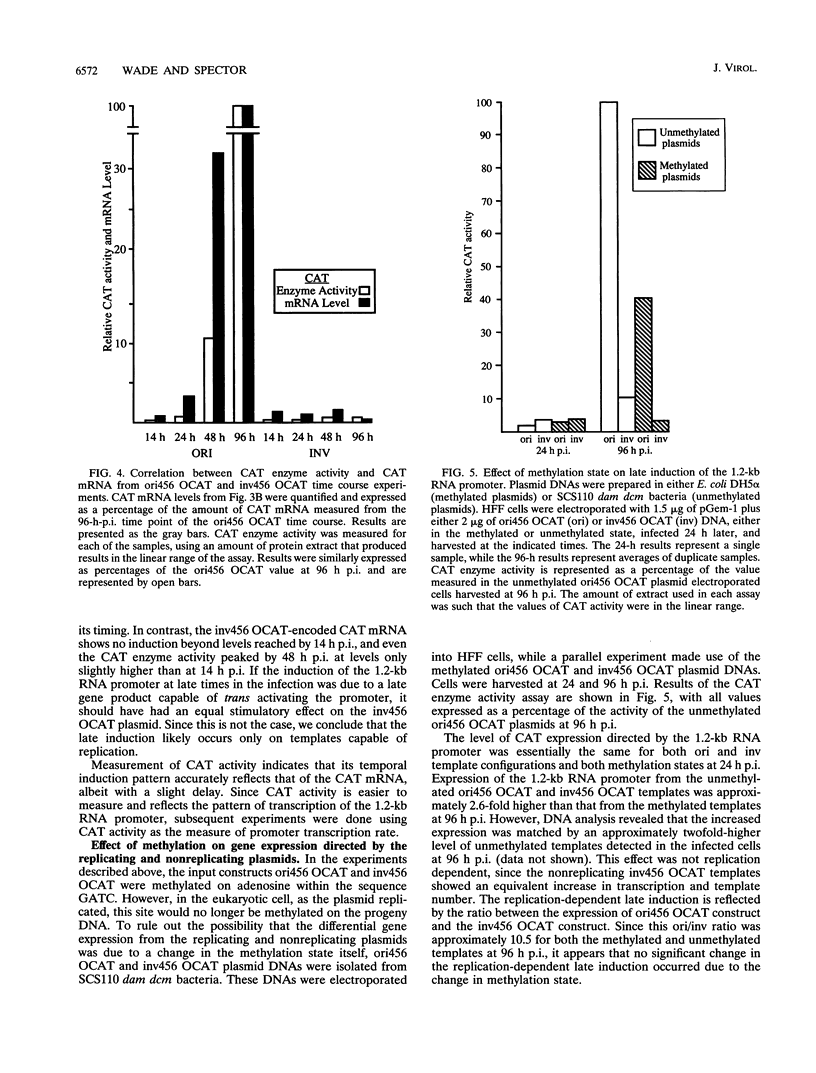
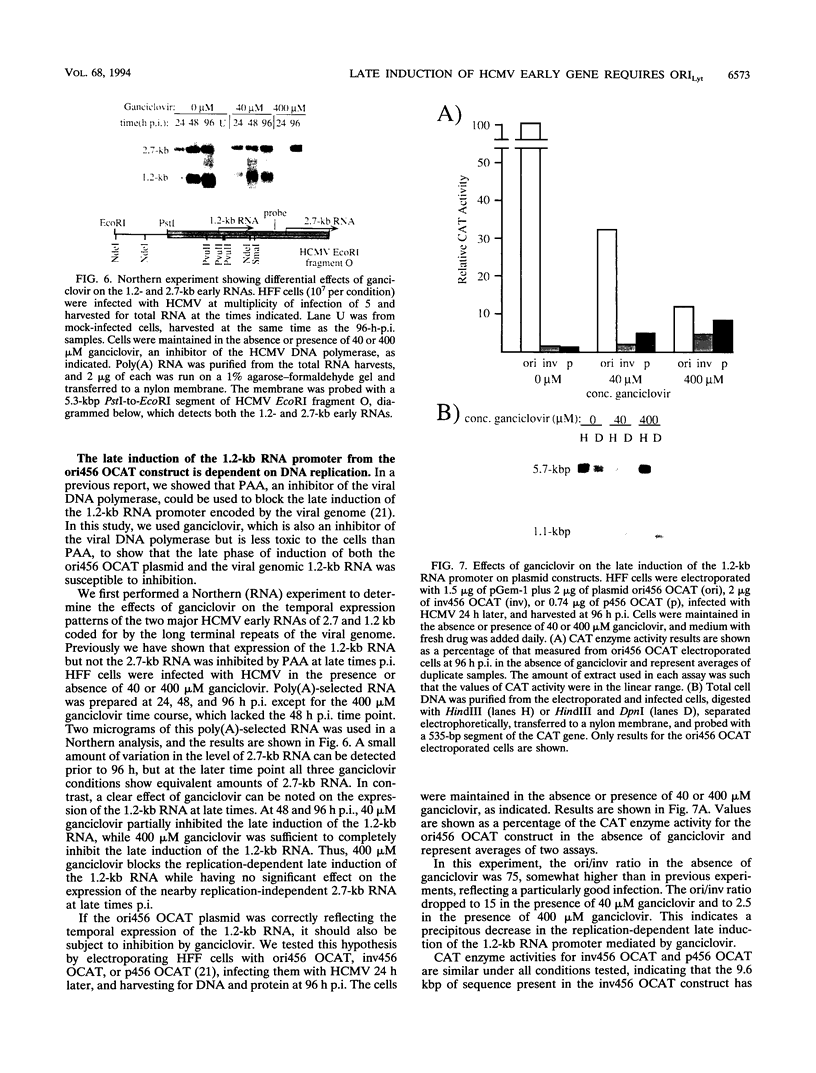
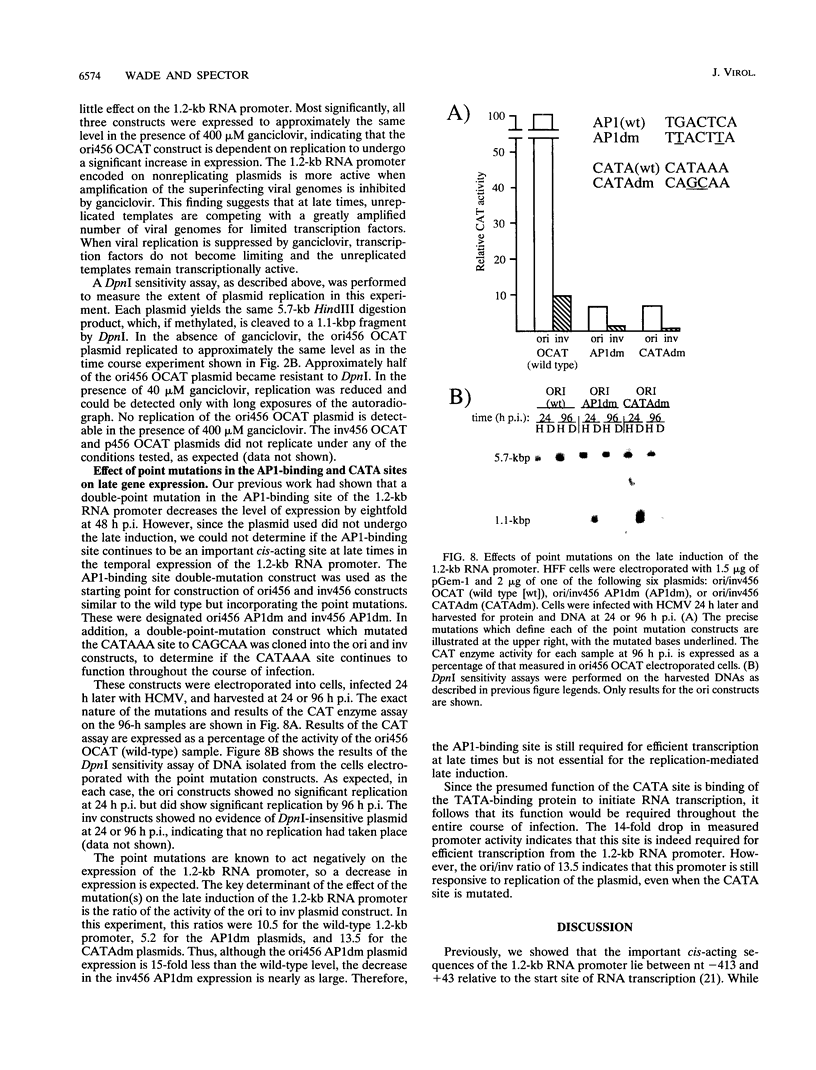
Plasmid constructs containing the 1.2-kb RNA promoter from the long terminal repeat region of human cytomegalovirus (HCMV) display the early-phase regulation of this promoter but lack the characteristic late induction (E. J. Wade, K. M. Klucher, and D. H. Spector, J. Virol. 66:2407-2417, 1992). To determine if the HCMV origin of replication (oriLyt) was necessary and sufficient for the late induction of the 1.2-kb RNA promoter, we cloned a 9.6-kbp segment of the origin of replication onto the p456 OCAT plasmid containing the 1.2-kb RNA promoter. This plasmid was designated ori456 OCAT. A control construct, which contains all of the same sequences as the ori456 OCAT construct except that a 2.4-kbp segment derived from HCMV EcoRI segment U is inverted in orientation to disrupt the origin function, was designated inv456 OCAT. After electroporation into human fibroblast cells and infection with HCMV 24 h later, ori456 OCAT replicated and showed the same early and late transcription pattern as the authentic viral 1.2-kb RNA. Under similar conditions, the inv456 OCAT neither replicated nor showed late induction. Experiments using plasmids synthesized in bacteria lacking methylation activity demonstrated that the late induction was not dependent on the change in methylation state of the plasmids. Ganciclovir, an inhibitor of the HCMV DNA polymerase, was used to demonstrate the replication dependence of the expression of the virally encoded 1.2-kb RNA, while the nearby early 2.7-kb RNA was unaffected. Ganciclovir also inhibited the late induction of the chloramphenicol acetyltransferase gene from ori456 OCAT, while expression from inv456 OCAT increased. Site-specific mutations in two previously identified important regulatory elements of the 1.2-kb RNA promoter, the AP1-binding site and the CATA site, indicated that these sites continue to contribute to promoter activity at late times but that the replication-dependent late induction acts independently of these sites. Possible mechanisms underlying the late induction are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G., Kacica M. A., Pari G., Punturieri S. M. Boundaries and structure of human cytomegalovirus oriLyt, a complex origin for lytic-phase DNA replication. J Virol. 1992 Jun;66(6):3373–3384. doi: 10.1128/jvi.66.6.3373-3384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S., Gibson W., Hayward G. S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990 Dec;64(12):6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen D. R., Kassavetis G. A., Barry J., Alberts B. M., Geiduschek E. P. Enhancement of bacteriophage T4 late transcription by components of the T4 DNA replication apparatus. Science. 1989 Sep 1;245(4921):952–958. doi: 10.1126/science.2672335. [DOI] [PubMed] [Google Scholar]
- Herendeen D. R., Kassavetis G. A., Geiduschek E. P. A transcriptional enhancer whose function imposes a requirement that proteins track along DNA. Science. 1992 May 29;256(5061):1298–1303. doi: 10.1126/science.1598572. [DOI] [PubMed] [Google Scholar]
- Hutchinson N. I., Sondermeyer R. T., Tocci M. J. Organization and expression of the major genes from the long inverted repeat of the human cytomegalovirus genome. Virology. 1986 Nov;155(1):160–171. doi: 10.1016/0042-6822(86)90176-5. [DOI] [PubMed] [Google Scholar]
- Hutchinson N. I., Tocci M. J. Characterization of a major early gene from the human cytomegalovirus long inverted repeat; predicted amino acid sequence of a 30-kDa protein encoded by the 1.2-kb mRNA. Virology. 1986 Nov;155(1):172–182. doi: 10.1016/0042-6822(86)90177-7. [DOI] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankelevich S., Kolman J. L., Bodnar J. W., Miller G. A nuclear matrix attachment region organizes the Epstein-Barr viral plasmid in Raji cells into a single DNA domain. EMBO J. 1992 Mar;11(3):1165–1176. doi: 10.1002/j.1460-2075.1992.tb05157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Everett R. D. DNA replication is required for abundant expression of a plasmid-borne late US11 gene of herpes simplex virus type 1. Nucleic Acids Res. 1986 May 12;14(9):3609–3625. doi: 10.1093/nar/14.9.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher K. M., Rabert D. K., Spector D. H. Sequences in the human cytomegalovirus 2.7-kilobase RNA promoter which mediate its regulation as an early gene. J Virol. 1989 Dec;63(12):5334–5343. doi: 10.1128/jvi.63.12.5334-5343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse M. J., Karlin S., Schachtel G. A., Mocarski E. S. Human cytomegalovirus origin of DNA replication (oriLyt) resides within a highly complex repetitive region. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5246–5250. doi: 10.1073/pnas.89.12.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- McDonough S. H., Staprans S. I., Spector D. H. Analysis of the major transcripts encoded by the long repeat of human cytomegalovirus strain AD169. J Virol. 1985 Mar;53(3):711–718. doi: 10.1128/jvi.53.3.711-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankervis G. A., Kumar M. L. Diseases produced by cytomegaloviruses. Med Clin North Am. 1978 Sep;62(5):1021–1035. doi: 10.1016/s0025-7125(16)31752-7. [DOI] [PubMed] [Google Scholar]
- Snowden B. W., Blair E. D., Wagner E. K. Transcriptional activation with concurrent or nonconcurrent template replication has differential effects on transient expression from herpes simplex virus promoters. Virus Genes. 1989 Mar;2(2):129–145. doi: 10.1007/BF00315257. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade E. J., Klucher K. M., Spector D. H. An AP-1 binding site is the predominant cis-acting regulatory element in the 1.2-kilobase early RNA promoter of human cytomegalovirus. J Virol. 1992 Apr;66(4):2407–2417. doi: 10.1128/jvi.66.4.2407-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. W., Akrigg A., Greenaway P. J. Transcription of the immediate early genes of human cytomegalovirus strain AD169. Virus Res. 1984;1(2):101–106. doi: 10.1016/0168-1702(84)90067-4. [DOI] [PubMed] [Google Scholar]