Abstract
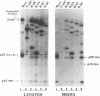
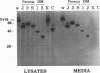
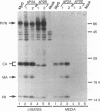
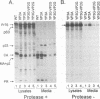
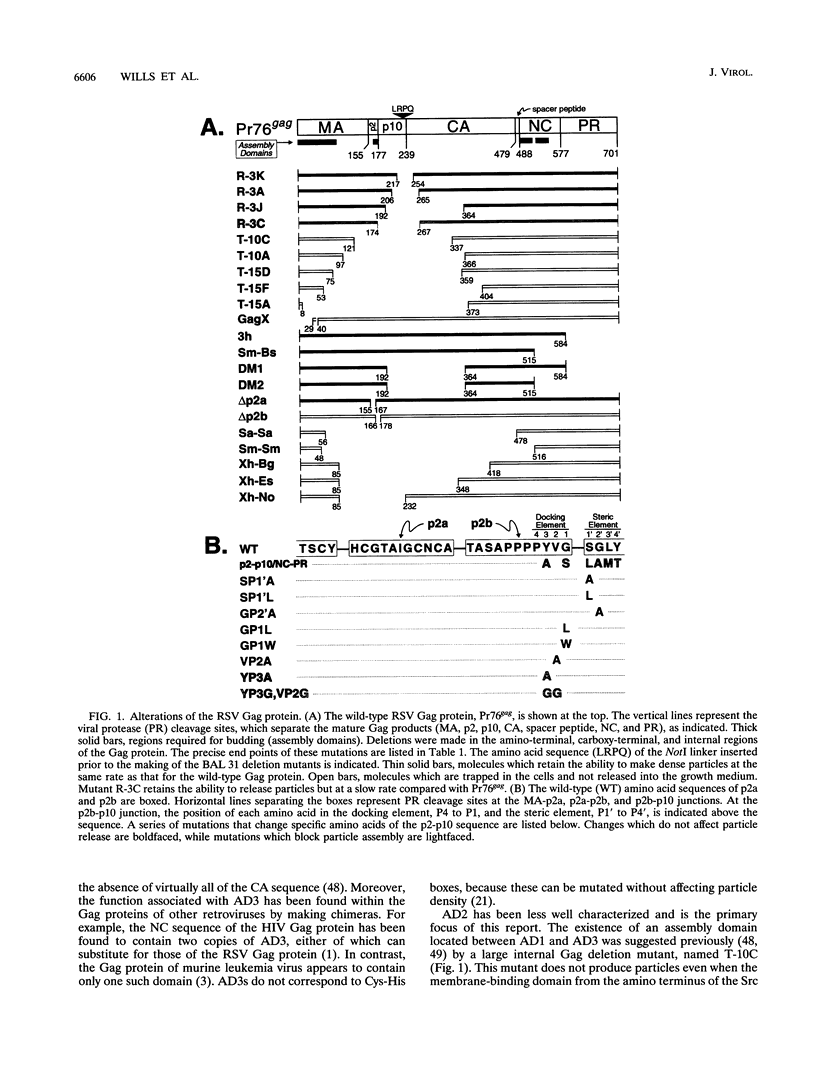
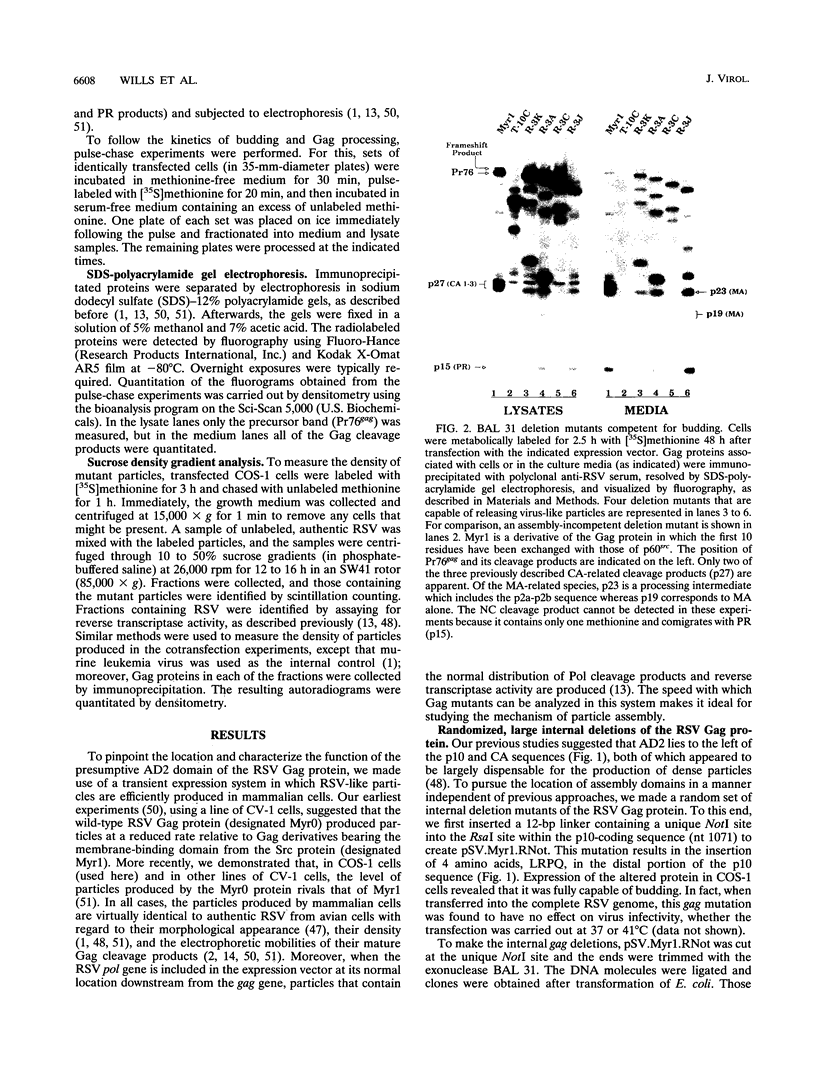
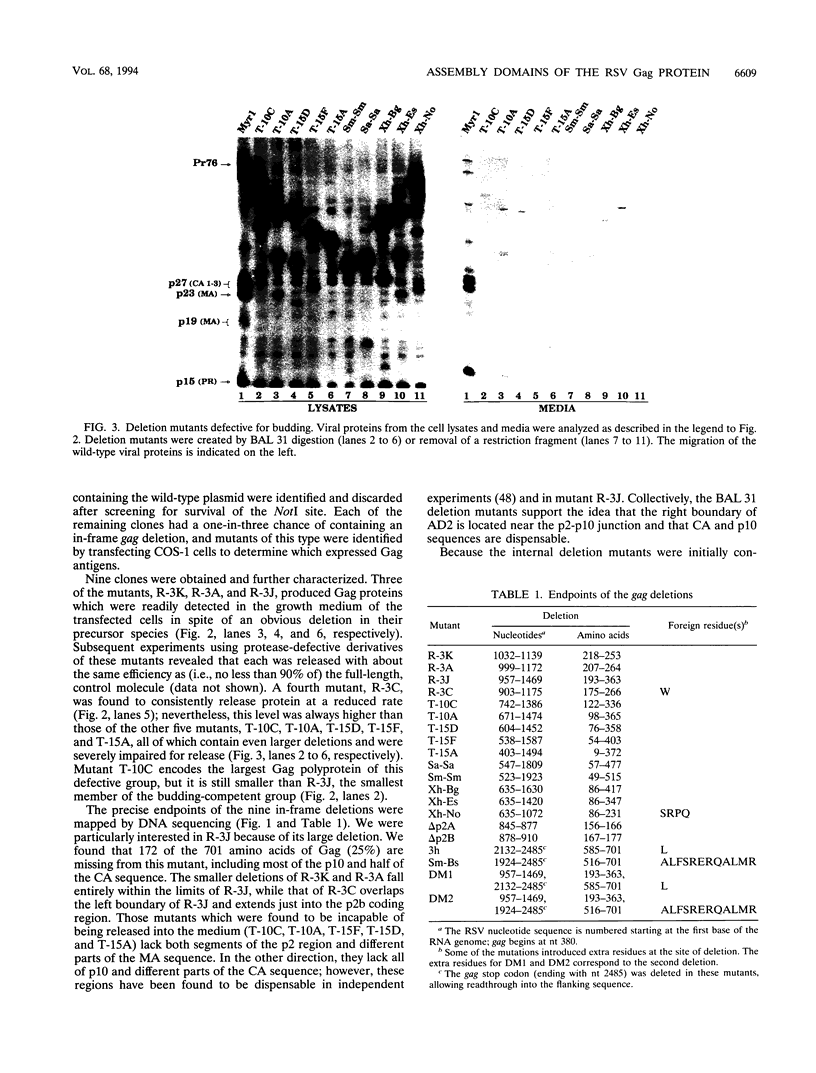
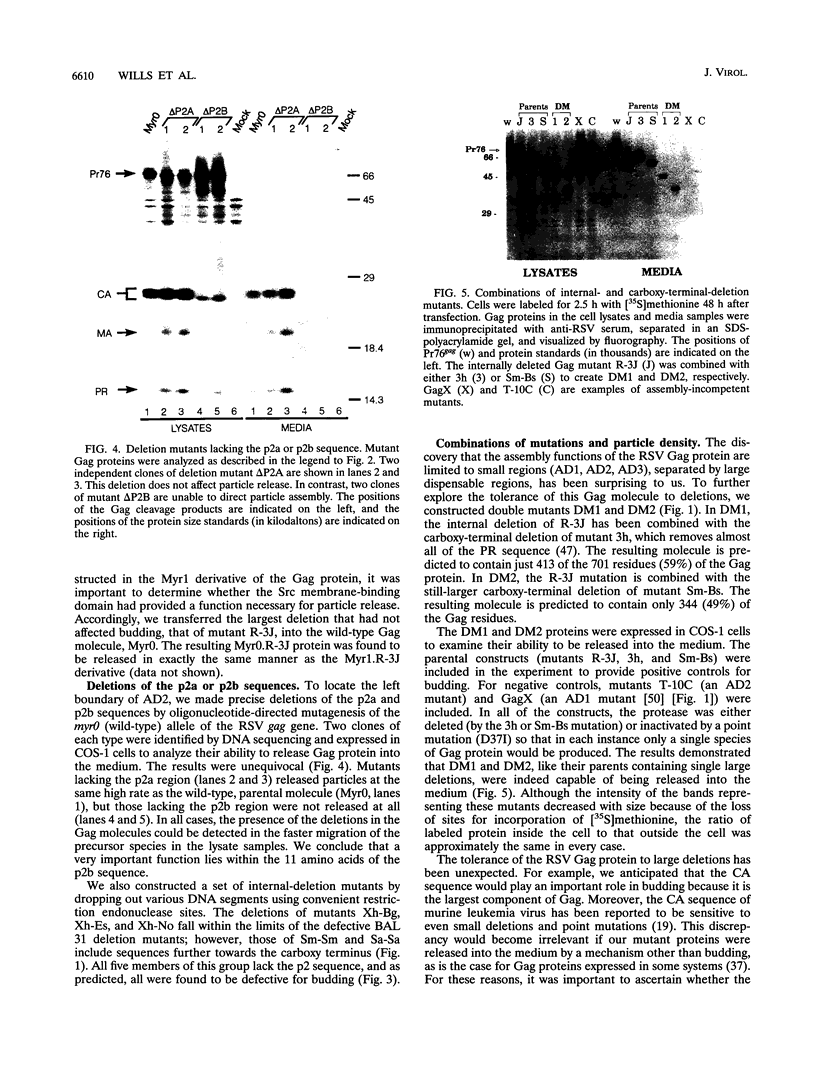
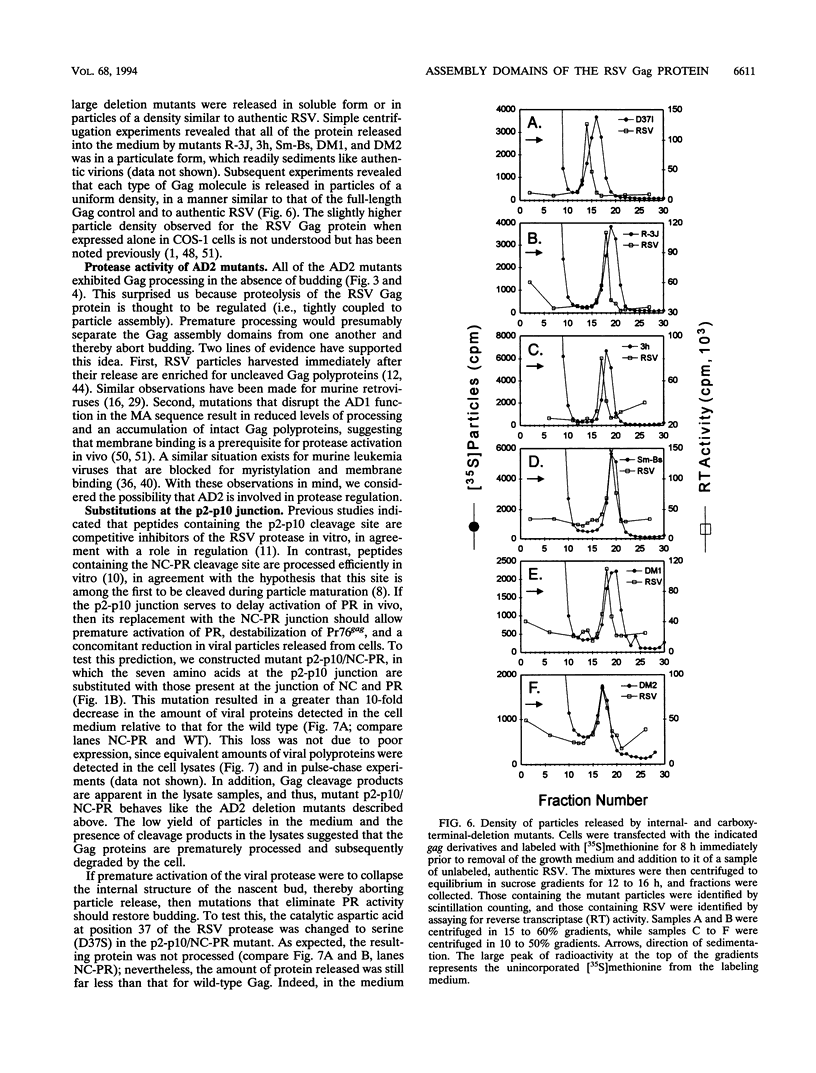
The Gag protein of Rous sarcoma virus has the ability to direct particle assembly at the plasma membrane in the absence of all the other virus-encoded components. An extensive deletion analysis has revealed that very large regions of this protein can be deleted without impairing budding and has suggested that the essential functions map to three discrete regions. In the studies reported here, we establish the location of assembly domain 2 (AD2) within the proline-rich p2b sequence of this Gag protein. AD2 mutants lacking the p2b sequence were completely defective for particle release even though their Gag proteins were tightly associated with the membrane fraction and exhibited high levels of protease activity. Mutations that inactivate the viral protease did not restore budding to wild-type levels for these mutants, indicating that the defect is not due simply to a loss of protease regulation. AD2 mutants could be rescued into dense particles in genetic complementation assays, indicating that their defect is not due to a gross alteration of the overall conformation of the protein and that the assembly function is not needed on every Gag molecule in the population. Several mutants with amino acid substitutions in the p2b sequence were found to have an intermediate capacity for budding. Inactivation of the protease of these mutants stabilized the Gag polyprotein within the cells and allowed an increase in particle release; however, the rate of budding remained slow. We favor the idea that AD2 is a dynamic region of movement, perhaps serving as a molecular hinge to allow the particle to emerge from the surface of the cell during budding.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett R. P., Nelle T. D., Wills J. W. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus gag proteins. J Virol. 1993 Nov;67(11):6487–6498. doi: 10.1128/jvi.67.11.6487-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. P., Rhee S., Craven R. C., Hunter E., Wills J. W. Amino acids encoded downstream of gag are not required by Rous sarcoma virus protease during gag-mediated assembly. J Virol. 1991 Jan;65(1):272–280. doi: 10.1128/jvi.65.1.272-280.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Weber I. T., Cameron C. E., Leis J. P., Skalka A. M. A range of catalytic efficiencies with avian retroviral protease subunits genetically linked to form single polypeptide chains. J Biol Chem. 1991 Mar 15;266(8):4951–4958. [PubMed] [Google Scholar]
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. I., Haggerty S., Dempsey M. P., Sharova N., Adzhubel A., Spitz L., Lewis P., Goldfarb D., Emerman M., Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993 Oct 14;365(6447):666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein H., Bizub D., Kotler M., Schatz G., Vogt V. M., Skalka A. M. Processing of avian retroviral gag polyprotein precursors is blocked by a mutation at the NC-PR cleavage site. J Virol. 1992 Mar;66(3):1781–1785. doi: 10.1128/jvi.66.3.1781-1785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein H., Bizub D., Skalka A. M. Assembly and processing of avian retroviral gag polyproteins containing linked protease dimers. J Virol. 1991 Nov;65(11):6165–6172. doi: 10.1128/jvi.65.11.6165-6172.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron C. E., Grinde B., Jacques P., Jentoft J., Leis J., Wlodawer A., Weber I. T. Comparison of the substrate-binding pockets of the Rous sarcoma virus and human immunodeficiency virus type 1 proteases. J Biol Chem. 1993 Jun 5;268(16):11711–11720. [PubMed] [Google Scholar]
- Cameron C. E., Grinde B., Jentoft J., Leis J., Weber I. T., Copeland T. D., Wlodawer A. Mechanism of inhibition of the retroviral protease by a Rous sarcoma virus peptide substrate representing the cleavage site between the gag p2 and p10 proteins. J Biol Chem. 1992 Nov 25;267(33):23735–23741. [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Craven R. C., Bennett R. P., Wills J. W. Role of the avian retroviral protease in the activation of reverse transcriptase during virion assembly. J Virol. 1991 Nov;65(11):6205–6217. doi: 10.1128/jvi.65.11.6205-6217.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R. C., Leure-duPree A. E., Erdie C. R., Wilson C. B., Wills J. W. Necessity of the spacer peptide between CA and NC in the Rous sarcoma virus gag protein. J Virol. 1993 Oct;67(10):6246–6252. doi: 10.1128/jvi.67.10.6246-6252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B., Parham P., Hill B. L. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell. 1990 Sep 7;62(5):875–887. doi: 10.1016/0092-8674(90)90263-e. [DOI] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz P., Spahr P. F. Analysis of deletions and thermosensitive mutations in Rous sarcoma virus gag protein p10. J Virol. 1993 Jul;67(7):3826–3834. doi: 10.1128/jvi.67.7.3826-3834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Lobel L. I. Mutants of murine leukemia viruses and retroviral replication. Biochim Biophys Acta. 1987 Jul 8;907(2):93–123. doi: 10.1016/0304-419x(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Gontarek R. R., McNally M. T., Beemon K. Mutation of an RSV intronic element abolishes both U11/U12 snRNP binding and negative regulation of splicing. Genes Dev. 1993 Oct;7(10):1926–1936. doi: 10.1101/gad.7.10.1926. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacostas V., Wolffe E. J., Nagashima K., Gonda M. A., Moss B. Overexpression of the HIV-1 gag-pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993 Apr;193(2):661–671. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G. Human immunodeficiency virus proteinase dimer as component of the viral polyprotein prevents particle assembly and viral infectivity. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3213–3217. doi: 10.1073/pnas.88.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G. Specific inhibitor of human immunodeficiency virus proteinase prevents the cytotoxic effects of a single-chain proteinase dimer and restores particle formation. J Virol. 1992 Jan;66(1):567–572. doi: 10.1128/jvi.66.1.567-572.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Bebenek K., McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Yoshinaka Y. Rauscher leukemia virus populations enriched for "immature" virions contain increased amounts of P70, the gag gene product. J Virol. 1978 Jan;25(1):416–421. doi: 10.1128/jvi.25.1.416-421.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergener K., Fäcke M., Welker R., Brinkmann V., Gelderblom H. R., Kräusslich H. G. Analysis of HIV particle formation using transient expression of subviral constructs in mammalian cells. Virology. 1992 Jan;186(1):25–39. doi: 10.1016/0042-6822(92)90058-w. [DOI] [PubMed] [Google Scholar]
- Pal R., Reitz M. S., Jr, Tschachler E., Gallo R. C., Sarngadharan M. G., Veronese F. D. Myristoylation of gag proteins of HIV-1 plays an important role in virus assembly. AIDS Res Hum Retroviruses. 1990 Jun;6(6):721–730. doi: 10.1089/aid.1990.6.721. [DOI] [PubMed] [Google Scholar]
- Peng C., Chang N. T., Chang T. W. Identification and characterization of human immunodeficiency virus type 1 gag-pol fusion protein in transfected mammalian cells. J Virol. 1991 May;65(5):2751–2756. doi: 10.1128/jvi.65.5.2751-2756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Mattaliano R. J., Vogt V. M. Structure and processing of the p2 region of avian sarcoma and leukemia virus gag precursor polyproteins. J Virol. 1986 Apr;58(1):50–58. doi: 10.1128/jvi.58.1.50-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer M., Hong S. S., Gay B., Cerutti M., Boulanger P. Expression and extracellular release of human immunodeficiency virus type 1 Gag precursors by recombinant baculovirus-infected cells. J Virol. 1992 May;66(5):3230–3235. doi: 10.1128/jvi.66.5.3230-3235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakalian M., Wills J. W., Vogt V. M. Efficiency and selectivity of RNA packaging by Rous sarcoma virus Gag deletion mutants. J Virol. 1994 Sep;68(9):5969–5981. doi: 10.1128/jvi.68.9.5969-5981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Rein A. Unmyristylated Moloney murine leukemia virus Pr65gag is excluded from virus assembly and maturation events. J Virol. 1989 May;63(5):2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell. 1989 Mar 24;56(6):911–913. doi: 10.1016/0092-8674(89)90621-1. [DOI] [PubMed] [Google Scholar]
- Stewart L., Schatz G., Vogt V. M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990 Oct;64(10):5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Snyder P. N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975 Nov;16(5):1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon R. A., Jr, Erdie C. R., Oliver M. G., Wills J. W. Incorporation of chimeric gag protein into retroviral particles. J Virol. 1990 Sep;64(9):4169–4179. doi: 10.1128/jvi.64.9.4169-4179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon R. A., Jr, Wills J. W. Characterization of a small (25-kilodalton) derivative of the Rous sarcoma virus Gag protein competent for particle release. J Virol. 1993 Sep;67(9):5550–5561. doi: 10.1128/jvi.67.9.5550-5561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Weldon R. A., Jr, Nelle T. D., Erdie C. R. Suppression of retroviral MA deletions by the amino-terminal membrane-binding domain of p60src. J Virol. 1991 Jul;65(7):3804–3812. doi: 10.1128/jvi.65.7.3804-3812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Parent L. J., Wills J. W., Resh M. D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994 Apr;68(4):2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]