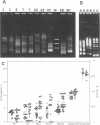
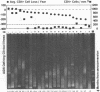
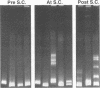
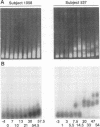
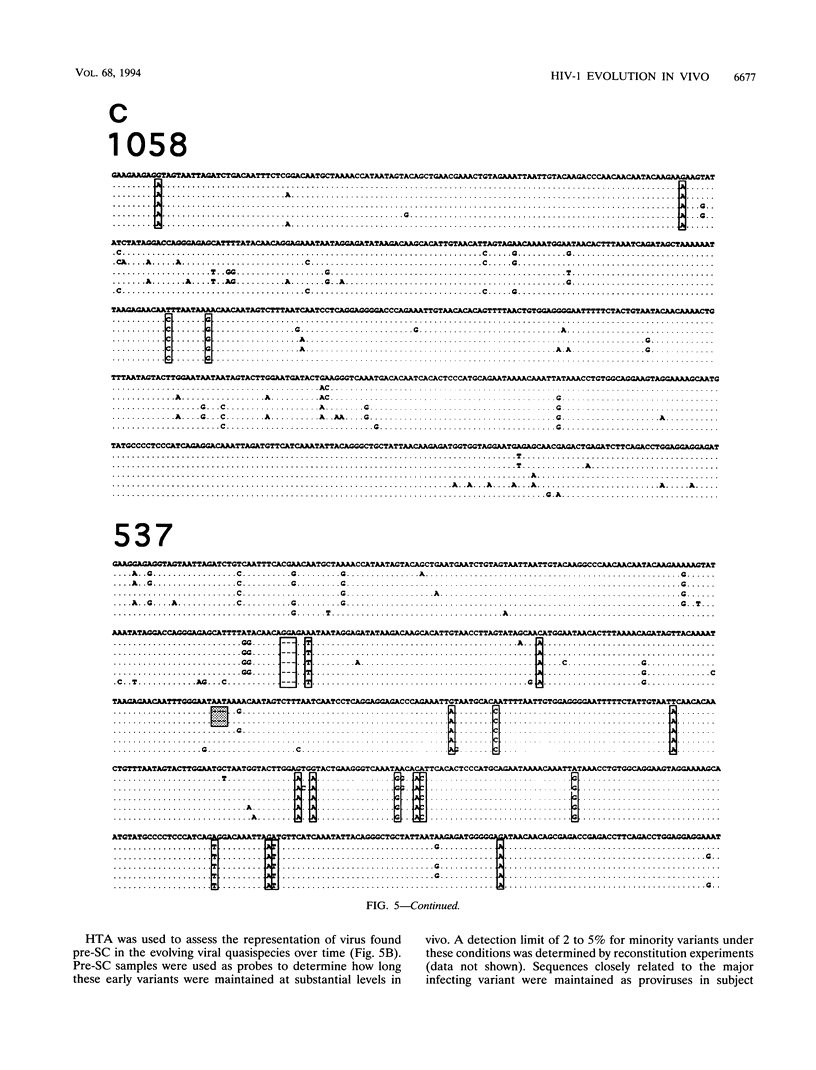
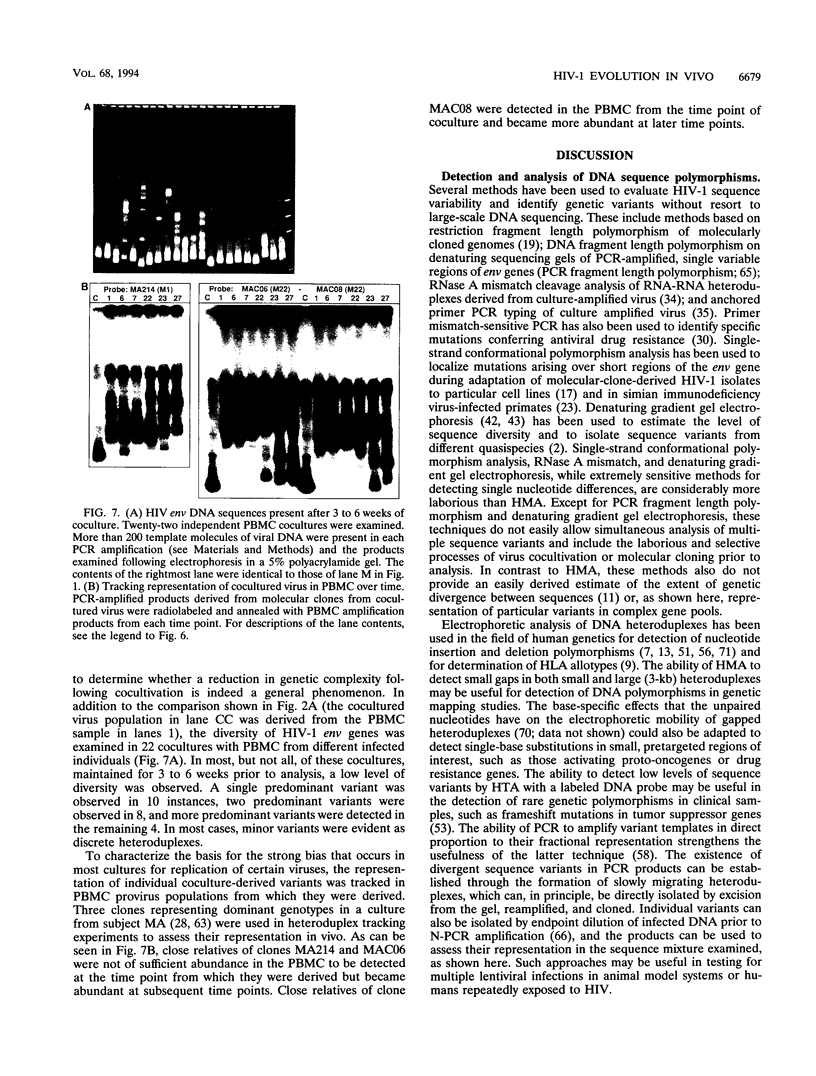
Abstract
High mutation rates and strong selective pressures imposed on human immunodeficiency viruses in vivo result in the formation of pools of genetic variants known as quasispecies. DNA heteroduplex mobility and tracking analyses were used to monitor the generation of HIV sequence diversity, to estimate quasispecies complexity, and to assess the turnover of genetic variants to approach an understanding of the relationship between viral quasispecies evolution in vivo and disease progression. Proviral DNA pools were nearly homogeneous soon after sexual transmission. The emergence and clearance of individual variants then occurred at different rates in different individuals. High quasispecies complexity was found in long-term-infected, asymptomatic individuals, while rapid CD4+ cell decline and AIDS were often, but not always, associated with lower quasispecies complexity. Proviral genetic variation was often low following in vitro culture, because of the outgrowth of one or a few variants that often became more abundant only later as proviruses in peripheral blood mononuclear cells. These studies provide insight into the dynamics of human immunodeficiency virus sequence changes in vivo and illustrate the utility of heteroduplex analysis for the study of phenomena associated with rapid genetic changes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Abrahamsson B., Nagy K., Aurelius E., Gaines H., Nyström G., Fenyö E. M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990 Feb;4(2):107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Andersson B., Ying J. H., Lewis D. E., Gibbs R. A. Rapid characterization of HIV-1 sequence diversity using denaturing gradient gel electrophoresis and direct automated DNA sequencing of PCR products. PCR Methods Appl. 1993 May;2(4):293–300. doi: 10.1101/gr.2.4.293. [DOI] [PubMed] [Google Scholar]
- Arendrup M., Nielsen C., Hansen J. E., Pedersen C., Mathiesen L., Nielsen J. O. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J Acquir Immune Defic Syndr. 1992;5(3):303–307. [PubMed] [Google Scholar]
- Bhattacharyya A., Lilley D. M. The contrasting structures of mismatched DNA sequences containing looped-out bases (bulges) and multiple mismatches (bubbles). Nucleic Acids Res. 1989 Sep 12;17(17):6821–6840. doi: 10.1093/nar/17.17.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. L., Monaghan P. Evolution of the structural proteins of human immunodeficiency virus: selective constraints on nucleotide substitution. AIDS Res Hum Retroviruses. 1988 Dec;4(6):399–407. doi: 10.1089/aid.1988.4.399. [DOI] [PubMed] [Google Scholar]
- Burns D. P., Desrosiers R. C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991 Apr;65(4):1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S. P., Eng B., Kan Y. W., Chui D. H. A rapid and simple electrophoretic method for the detection of mutations involving small insertion or deletion: application to beta-thalassemia. Hum Genet. 1991 Oct;87(6):728–730. doi: 10.1007/BF00201734. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay T. M., Bidwell J. L., Howard M. R., Bradley B. A. PCR-fingerprinting for selection of HLA matched unrelated marrow donors. Collaborating Centres in the IMUST Study. Lancet. 1991 May 4;337(8749):1049–1052. doi: 10.1016/0140-6736(91)91704-x. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genetic diversity and evolution of retroviruses. Curr Top Microbiol Immunol. 1992;176:143–164. doi: 10.1007/978-3-642-77011-1_10. [DOI] [PubMed] [Google Scholar]
- Delwart E. L., Shpaer E. G., Louwagie J., McCutchan F. E., Grez M., Rübsamen-Waigmann H., Mullins J. I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993 Nov 19;262(5137):1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. Determination of retroviral mutation rates using spleen necrosis virus-based vectors and helper cells. Biotechnology. 1991;16:21–33. [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Faulkner D. V., Jurka J. Multiple aligned sequence editor (MASE). Trends Biochem Sci. 1988 Aug;13(8):321–322. doi: 10.1016/0968-0004(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Fenouillet E., Blanes N., Coutellier A., Demarquest J., Rozenbaum W., Gluckman J. C. Monitoring of antibodies against human immunodeficiency virus type 1 p25 core protein as prognostic marker. J Infect Dis. 1992 Sep;166(3):611–616. doi: 10.1093/infdis/166.3.611. [DOI] [PubMed] [Google Scholar]
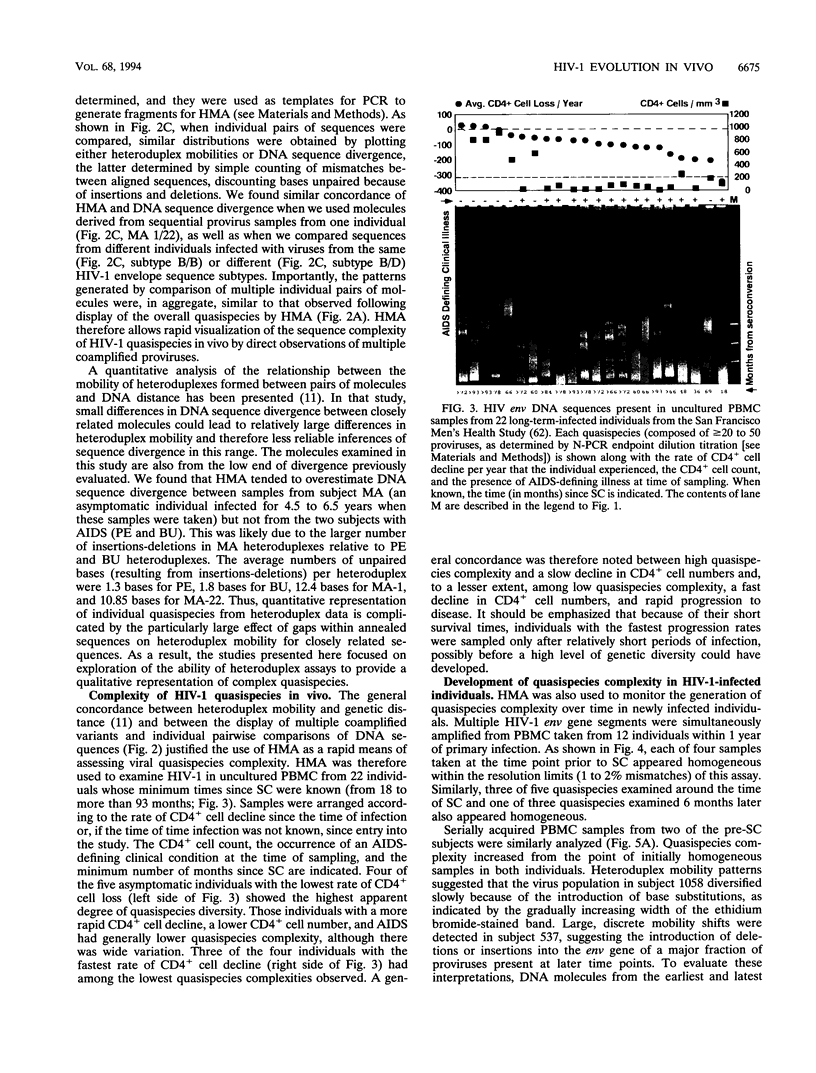
- Fouchier R. A., Groenink M., Kootstra N. A., Tersmette M., Huisman H. G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992 May;66(5):3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Silver J., Peden K. Changes in both gp120 and gp41 can account for increased growth potential and expanded host range of human immunodeficiency virus type 1. J Virol. 1992 Jul;66(7):4445–4451. doi: 10.1128/jvi.66.7.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink M., Fouchier R. A., Broersen S., Baker C. H., Koot M., van't Wout A. B., Huisman H. G., Miedema F., Tersmette M., Schuitemaker H. Relation of phenotype evolution of HIV-1 to envelope V2 configuration. Science. 1993 Jun 4;260(5113):1513–1516. doi: 10.1126/science.8502996. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
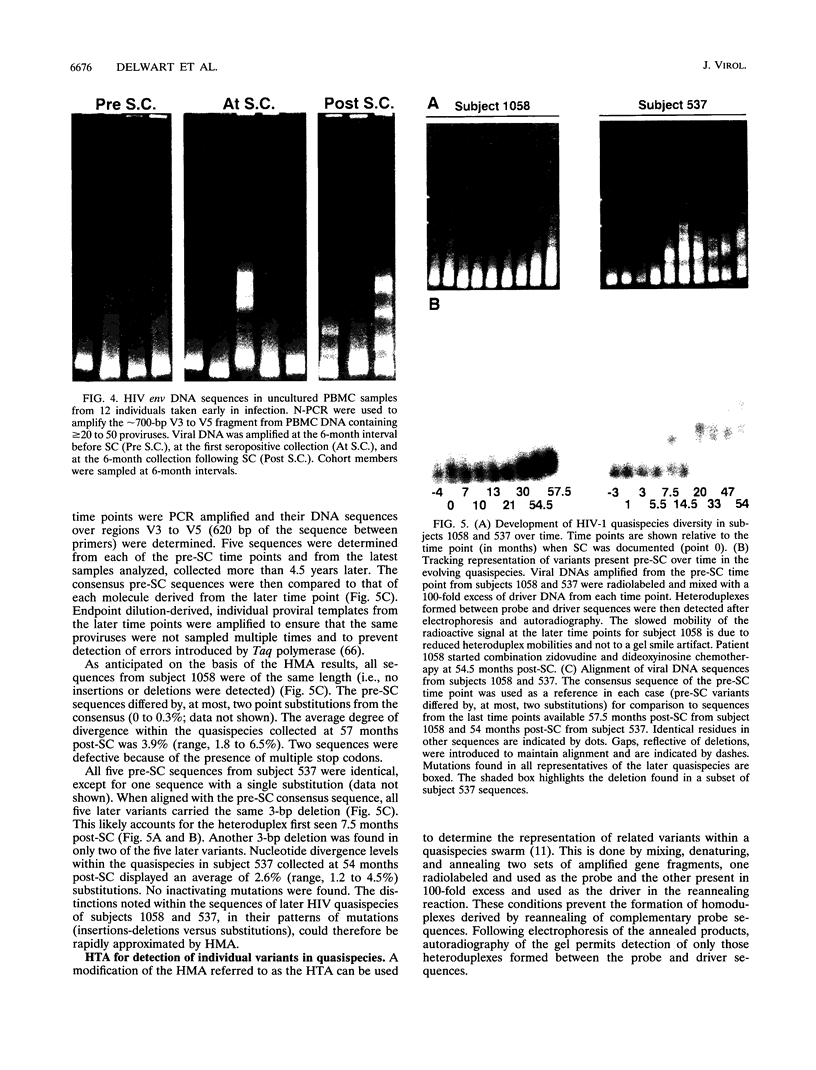
- Hirsch V. M., Edmondson P., Murphey-Corb M., Arbeille B., Johnson P. R., Mullins J. I. SIV adaptation to human cells. Nature. 1989 Oct 19;341(6243):573–574. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Resnick L., Dimarzoveronese F., Rota T. R., Hirsch M. S. Primary human T-lymphotropic virus type III infection. Ann Intern Med. 1985 Dec;103(6 ):880–883. doi: 10.7326/0003-4819-103-6-880. [DOI] [PubMed] [Google Scholar]
- Hynes N. A., Adger-Johnson D., Dapolito G., Hirsch V. M. Rapid screening for simian immunodeficiency virus variants using single-strand conformation polymorphism of PCR-amplified DNA fragments. AIDS Res Hum Retroviruses. 1993 Aug;9(8):803–806. doi: 10.1089/aid.1993.9.803. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Hamm T. E., Goldstein S., Kitov S., Hirsch V. M. The genetic fate of molecularly cloned simian immunodeficiency virus in experimentally infected macaques. Virology. 1991 Nov;185(1):217–228. doi: 10.1016/0042-6822(91)90769-8. [DOI] [PubMed] [Google Scholar]
- Keet I. P., Krijnen P., Koot M., Lange J. M., Miedema F., Goudsmit J., Coutinho R. A. Predictors of rapid progression to AIDS in HIV-1 seroconverters. AIDS. 1993 Jan;7(1):51–57. doi: 10.1097/00002030-199301000-00008. [DOI] [PubMed] [Google Scholar]
- Kuiken C. L., Zwart G., Baan E., Coutinho R. A., van den Hoek J. A., Goudsmit J. Increasing antigenic and genetic diversity of the V3 variable domain of the human immunodeficiency virus envelope protein in the course of the AIDS epidemic. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9061–9065. doi: 10.1073/pnas.90.19.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken C. L., de Jong J. J., Baan E., Keulen W., Tersmette M., Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992 Jul;66(7):4622–4627. doi: 10.1128/jvi.66.7.4622-4627.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang W., Perkins H., Anderson R. E., Royce R., Jewell N., Winkelstein W., Jr Patterns of T lymphocyte changes with human immunodeficiency virus infection: from seroconversion to the development of AIDS. J Acquir Immune Defic Syndr. 1989;2(1):63–69. [PubMed] [Google Scholar]
- Larder B. A., Kellam P., Kemp S. D. Zidovudine resistance predicted by direct detection of mutations in DNA from HIV-infected lymphocytes. AIDS. 1991 Feb;5(2):137–144. doi: 10.1097/00002030-199102000-00002. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Li Y., Kappes J. C., Conway J. A., Price R. W., Shaw G. M., Hahn B. H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991 Aug;65(8):3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson A. R., Buchbinder S. P., Sheppard H. W., Mawle A. C., Wilber J. C., Stanley M., Hart C. E., Hessol N. A., Holmberg S. D. Long-term human immunodeficiency virus infection in asymptomatic homosexual and bisexual men with normal CD4+ lymphocyte counts: immunologic and virologic characteristics. J Infect Dis. 1991 May;163(5):959–965. doi: 10.1093/infdis/163.5.959. [DOI] [PubMed] [Google Scholar]
- López-Galíndez C., Rojas J. M., Nájera R., Richman D. D., Perucho M. Characterization of genetic variation and 3'-azido-3'-deoxythymidine- resistance mutations of human immunodeficiency virus by the RNase A mismatch cleavage method. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4280–4284. doi: 10.1073/pnas.88.10.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan F. E., Sanders-Buell E., Oster C. W., Redfield R. R., Hira S. K., Perine P. L., Ungar B. L., Burke D. S. Genetic comparison of human immunodeficiency virus (HIV-1) isolates by polymerase chain reaction. J Acquir Immune Defic Syndr. 1991;4(12):1241–1250. [PubMed] [Google Scholar]
- McKeating J. A., Gow J., Goudsmit J., Pearl L. H., Mulder C., Weiss R. A. Characterization of HIV-1 neutralization escape mutants. AIDS. 1989 Dec;3(12):777–784. doi: 10.1097/00002030-198912000-00001. [DOI] [PubMed] [Google Scholar]
- McNearney T., Hornickova Z., Markham R., Birdwell A., Arens M., Saah A., Ratner L. Relationship of human immunodeficiency virus type 1 sequence heterogeneity to stage of disease. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10247–10251. doi: 10.1073/pnas.89.21.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990 Apr 11;18(7):1687–1691. doi: 10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Maniatis T., Lerman L. S. Modification of the melting properties of duplex DNA by attachment of a GC-rich DNA sequence as determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3111–3129. doi: 10.1093/nar/13.9.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Smit L., Dunlop N., Hatch W., Merges M., Waters D., Kelliher J., Gallo R. C., Fischinger P. J., Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990 Aug;64(8):3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. A., Anderson R. M., McLean A. R., Wolfs T. F., Goudsmit J., May R. M. Antigenic diversity thresholds and the development of AIDS. Science. 1991 Nov 15;254(5034):963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M. Alterations in potential sites for glycosylation predominate during evolution of the simian immunodeficiency virus envelope gene in macaques. J Virol. 1992 Oct;66(10):5937–5948. doi: 10.1128/jvi.66.10.5937-5948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M., Papenhausen M. D., Benveniste R. E., Morton W. R. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991 Dec;65(12):7025–7031. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Shlesinger Y., Daar E. S., Moudgil T., Ho D. D., Chen I. S. Rapid generation of sequence variation during primary HIV-1 infection. AIDS. 1992 May;6(5):453–460. doi: 10.1097/00002030-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Pathak V. K., Temin H. M. Broad spectrum of in vivo forward mutations, hypermutations, and mutational hotspots in a retroviral shuttle vector after a single replication cycle: substitutions, frameshifts, and hypermutations. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6019–6023. doi: 10.1073/pnas.87.16.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paw B. H., Tieu P. T., Kaback M. M., Lim J., Neufeld E. F. Frequency of three Hex A mutant alleles among Jewish and non-Jewish carriers identified in a Tay-Sachs screening program. Am J Hum Genet. 1990 Oct;47(4):698–705. [PMC free article] [PubMed] [Google Scholar]
- Phillips A. N., Lee C. A., Elford J., Janossy G., Timms A., Bofill M., Kernoff P. B. Serial CD4 lymphocyte counts and development of AIDS. Lancet. 1991 Feb 16;337(8738):389–392. doi: 10.1016/0140-6736(91)91166-r. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Wilson C., Naugle C., Gallo R. C., Robert-Guroff M. Generation of a neutralization-resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell. 1988 Jul 1;54(1):57–63. doi: 10.1016/0092-8674(88)90179-1. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Bebenek K., Kunkel T. A. The accuracy of reverse transcriptase from HIV-1. Science. 1988 Nov 25;242(4882):1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- Rommens J., Kerem B. S., Greer W., Chang P., Tsui L. C., Ray P. Rapid nonradioactive detection of the major cystic fibrosis mutation. Am J Hum Genet. 1990 Feb;46(2):395–396. [PMC free article] [PubMed] [Google Scholar]
- Roos M. T., Lange J. M., de Goede R. E., Coutinho R. A., Schellekens P. T., Miedema F., Tersmette M. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis. 1992 Mar;165(3):427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- Ruano G., Kidd K. K. Modeling of heteroduplex formation during PCR from mixtures of DNA templates. PCR Methods Appl. 1992 Nov;2(2):112–116. doi: 10.1101/gr.2.2.112. [DOI] [PubMed] [Google Scholar]
- Schellekens P. T., Tersmette M., Roos M. T., Keet R. P., de Wolf F., Coutinho R. A., Miedema F. Biphasic rate of CD4+ cell count decline during progression to AIDS correlates with HIV-1 phenotype. AIDS. 1992 Jul;6(7):665–669. doi: 10.1097/00002030-199207000-00008. [DOI] [PubMed] [Google Scholar]
- Schuitemaker H., Koot M., Kootstra N. A., Dercksen M. W., de Goede R. E., van Steenwijk R. P., Lange J. M., Schattenkerk J. K., Miedema F., Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992 Mar;66(3):1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard H. W., Ascher M. S., McRae B., Anderson R. E., Lang W., Allain J. P. The initial immune response to HIV and immune system activation determine the outcome of HIV disease. J Acquir Immune Defic Syndr. 1991;4(7):704–712. [PubMed] [Google Scholar]
- Sheppard H. W., Lang W., Ascher M. S., Vittinghoff E., Winkelstein W. The characterization of non-progressors: long-term HIV-1 infection with stable CD4+ T-cell levels. AIDS. 1993 Sep;7(9):1159–1166. [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Ludlam C. A., Bishop J. O., Brown A. J. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J Virol. 1990 Dec;64(12):5840–5850. doi: 10.1128/jvi.64.12.5840-5850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., McOmish F., Balfe P., Ludlam C. A., Brown A. J. Discontinuous sequence change of human immunodeficiency virus (HIV) type 1 env sequences in plasma viral and lymphocyte-associated proviral populations in vivo: implications for models of HIV pathogenesis. J Virol. 1991 Nov;65(11):6266–6276. doi: 10.1128/jvi.65.11.6266-6276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S. Viral burden in AIDS. Nature. 1993 Nov 4;366(6450):22–22. doi: 10.1038/366022b0. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Griffith J. Effects of bulge composition and flanking sequence on the kinking of DNA by bulged bases. Biochemistry. 1991 Feb 5;30(5):1358–1363. doi: 10.1021/bi00219a028. [DOI] [PubMed] [Google Scholar]
- White M. B., Carvalho M., Derse D., O'Brien S. J., Dean M. Detecting single base substitutions as heteroduplex polymorphisms. Genomics. 1992 Feb;12(2):301–306. doi: 10.1016/0888-7543(92)90377-5. [DOI] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992 Jul;189(1):103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- Wolfs T. F., de Jong J. J., Van den Berg H., Tijnagel J. M., Krone W. J., Goudsmit J. Evolution of sequences encoding the principal neutralization epitope of human immunodeficiency virus 1 is host dependent, rapid, and continuous. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9938–9942. doi: 10.1073/pnas.87.24.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky S. M., Wike C. M., Korber B. T., Hutto C., Parks W. P., Rosenblum L. L., Kunstman K. J., Furtado M. R., Muñoz J. L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992 Feb 28;255(5048):1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Zhang L. Q., MacKenzie P., Cleland A., Holmes E. C., Brown A. J., Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993 Jun;67(6):3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Mo H., Wang N., Nam D. S., Cao Y., Koup R. A., Ho D. D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993 Aug 27;261(5125):1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]