Abstract
The vascular endothelial growth factor (VEGF) has been shown to be a significant mediator of angiogenesis during a variety of normal and pathological processes, including tumor development. Human U87MG glioblastoma cells express the three VEGF isoforms: VEGF121, VEGF165, and VEGF189. Here, we have investigated whether these three isoforms have distinct roles in glioblastoma angiogenesis. Clones that overexpressed each isoform were derived and inoculated into mouse brains. Mice that received VEGF121- and VEGF165-overexpressing cells developed intracerebral hemorrhages after 60–90 hr. In contrast, mice implanted with VEGF189-overexpressing cells had only slightly larger tumors than those caused by parental cells and little evidence of hemorrhage at these early times after implantation, whereas, after longer periods of growth, enhanced angiogenicity and tumorigenicity were apparent. There was rapid blood vessel growth and breakdown around the tumors caused by cells overexpressing VEGF121 and VEGF165, whereas there was similar vascularization but no eruption in the vicinity of those tumors caused by cells overexpressing VEGF189, and none on the border of the tumors caused by the parental cells. Thus, by introducing VEGF-overexpressing glioblastoma cells into the brain, we have established a reproducible and predictable in vivo model of tumor-associated intracerebral hemorrhage caused by the enhanced expression of single molecular species. Such a model should be useful for uncovering the role of VEGF isoforms in the mechanisms of angiogenesis and for investigating intracerebral hemorrhage due to ischemic stroke or congenital malformations.
Keywords: angiogenesis, glioblastoma
Intracerebral hemorrhage is a rupture of blood vessels that results in the release of blood cells and substances into the surrounding tissues. Hemorrhage can result from hypertension, cerebral amyloid angioplasty, ruptured saccular aneurysms, arteriovenous malformations, hemorrhagic disorders, drug abuse, anticoagulation, and brain tumors (1). In the latter case, intracerebral hemorrhage can occur upon development of glioblastoma multiforme or oligodendroglioma (2). Glioblastomas are among the most heavily neovascularized neoplasms (3), and it has been shown that vascular endothelial growth factor (VEGF) and its receptors, Flt-1 and Flk-1/KDR, play a major role in their angiogenesis (4). VEGF is a 34- to 42-kDa heparin-binding, dimeric, disulfide-bound glycoprotein and exists as five spliced isoforms having 121, 145, 165, 189, and 206 amino acids, respectively. In contrast to basic fibroblast growth factor, another major angiogenic factor, it has a typical signal peptide composed of 26 amino acids and is efficiently secreted from cells (5, 6).
In addition to having pivotal roles in vasculogenesis and angiogenesis (7), accumulated evidence suggests that VEGF may also be a major factor in several pathological processes. For example, levels of VEGF were increased in ocular fluids of patients with proliferative diabetic retinopathy and other retinal disorders (8), which often show angiogenesis and hemorrhage (2). Similarly, hemorrhage that is associated with perfusion of the cerebral vasculature after ischemic infarction is a significant clinical entity that limits the use of tissue plasminogen activator in treating patients with ischemic stroke (9). VEGF expression has also been shown to increase dramatically within 6–12 hr of retinal hypoxia and to remain elevated until the development of neovascularization in a mouse model of proliferative retinopathy (10). VEGF acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity (11). Elevated expression of VEGF and its two receptors, Flt-1 and KDR, has also been demonstrated in rheumatoid synovial tissues (12), and VEGF is up-regulated by the myocardial ischemia that develops as a result of epicardial coronary obstruction (13). Immunologically detectable VEGF was apparent in several types of diseases and tumors of the human pediatric and adult central nervous systems, including vascular malformations and aneurysms (14, 15) and meningiomas (16) as well as hemangioblastomas (17). However, the molecular mechanisms by which VEGF mediates the development of these pathological disorders has remained obscure.
Here, we report that the single overexpression of either of the VEGF isoforms VEGF121 or VEGF165 by human U87MG glioblastoma cells causes reproducible and predictable intracerebral hemorrhage. Several individual U87MG cell clones were isolated that showed highly increased VEGF121 or VEGF165 protein secretion and elevated abilities to stimulate migration of aortic endothelial cells expressing the VEGF receptor, KDR. When such VEGF-overexpressing cells were stereotactically implanted into mouse brains, intracerebral hemorrhage developed rapidly within 60–90 hr. In contrast, there was no indication of hemorrhage in mice that received either VEGF189-overexpressing or parental U87MG cells. Rather, enhanced angiogenicity and tumorigenicity compared with that elicited by parental cells was observed in mice that received VEGF189-overexpressing cells upon extended growth periods. These results suggest a novel function for VEGF in the development of tumor-associated intracerebral hemorrhage and provide an in vivo model with which to determine the molecular mechanisms of angiogenesis and the distinct roles of different VEGF isoforms in the process.
MATERIALS AND METHODS
Cell Lines and Tissue Culture.
U87MG cells and their culture were described previously (18). Porcine aortic endothelial cells that express human KDR (PAE/KDR, a gift from Lena Claesson-Welsh, Ludwig Institute for Cancer Research, Uppsala, Sweden) were cultured in Ham’s F-12 medium containing 10% fetal calf serum (Sigma). The cells were starved in 0.1% BSA/F-12 for 14–16 hr before they were used for in vitro migration assays.
Selection of Clones from the U87MG Cells Transfected with the VEGF-Expression Constructs, Conditioned Media (CM), and VEGF ELISA.
These experiments were carried out as described (18).
Western Blot Analysis and Heparin Enrichment of VEGF from CM.
Samples containing 30 μg of total protein from total cell lysates were separated in an SDS/12.5% polyacrylamide gel and transferred onto a nitrocellulose membrane (Bio-Rad). The rinsed and blocked membrane was then incubated with a mouse monoclonal anti-VEGF antibody (clone G153–341; PharMingen) at room temperature for 1 hr. The blot was washed and probed with a rabbit anti-mouse antibody, conjugated with horseradish peroxidase (Dako) at room temperature for 20 min. The blot was washed again and developed with ECL (enhanced chemiluminescence) reagents (Amersham).
Heparin enrichment of VEGF was done with the CM from the parental U87MG and VEGF165- and VEGF189-overexpressing cells. The CM was generated in a way similar to that described above. After 48 hr, the CM was normalized and the final volumes were adjusted to 2 ml with media. The preabsorbed CM was transferred into new tubes that contained preswollen heparin-Sepharose 6L (Pharmacia). The tubes were rotated for 1 hr at 4°C. The heparin-Sepharose–protein complexes were spun down, washed, and analyzed by VEGF Western blot analysis.
Endothelial Cell Migration Assay.
The assays were similar to those described in ref. 18 except that the endothelial cells used were PAE/KDR and the collected CM was diluted 1:100 in 0.5% BSA/Ham’s F-12 for the assays.
Intracerebral Stereotactic Implantation and Mouse Brain Tissue Fixation.
Intracerebral stereotactic implantation and preparation of frozen brain samples were performed as described (18). Some samples were fixed in 10% formalin/PBS overnight followed by dehydration and paraffin embedding.
Immunohistochemical Analysis of Mouse Brain Samples.
To view hemorrhagic mouse brains or tumors in the mouse brain, paraffin sections (5 μm) were stained with hematoxylin and eosin. Immunohistochemical analysis of cryostat sections and quantitative analysis of the blood vessel densities of tumor samples was performed as described (18). The antibodies used in the analysis were an anti-CD31 mouse monoclonal antibody (clone MEC 13.3, PharMingen) and its isotype control antibody (PharMingen).
RESULTS
Overexpression of VEGF in U87MG Cells.
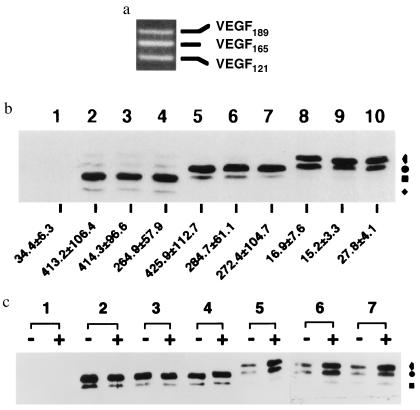
Human U87MG glioblastoma cells endogeneously express three VEGF splice variants, VEGF121, VEGF165, and VEGF189 in roughly equal amounts (Fig. 1a); these PCR-based observations were corroborated by using RNase-protection assays (data not shown). We sought to determine whether there are biologically functional differences among these variants by overexpressing these isoforms individually in cells. Constructs containing each isoform under human cytomegalovirus (CMV) promoter control were assembled and separately transfected into U87MG cells, and single hygromycin B-resistant cells were isolated as described previously (18). Eight to 10 clones that overexpressed each of the VEGF protein isoforms were identified by Western blot analysis (Fig. 1b). The secretion of the VEGF proteins by these clones was further assessed by VEGF ELISA (Fig. 1b) and by enrichment of CM after treating the cells with heparin (Fig. 1c). Fig. 1b shows the levels of VEGF expression in three examples from each isoform-overexpressing clone class: clones 7, 12, and 19 for VEGF121; clones 2, 15, and 31 for VEGF165; and clones 10, 26, and 30 for VEGF189. Each clone expressed high levels of its VEGF protein compared with parental U87MG cells (Fig. 1 b and c). Moreover, each of the VEGF121-and VEGF165-overexpressing clones secreted VEGF proteins into their growth medium at levels that were 7- to 12-fold higher than parental U87MG cells (Fig. 1b). The secretion of the three VEGF isoforms from overexpressing cells was qualitatively consistent with an earlier report (19), which showed VEGF121 and VEGF165 proteins to be efficiently secreted while VEGF189 protein was not, as it was mainly bound to the extracellular matrix. Exposure of the cells to heparin caused VEGF189 protein to be released into the CM (Fig. 1c). The antibody used in the VEGF ELISA assay did not react with VEGF189 protein, thus precluding its measurement in this way. The overexpression of each isoform of VEGF had no obvious effect on cell growth in vitro, as the growth rates of each of the clones described here were similar (data not shown).
Figure 1.
Expression of three isoforms of VEGF in human U87MG glioblastoma cells. (a) Reverse transcription PCR of VEGF from total RNA isolated from U87MG cells. cDNA lengths for VEGF121, VEGF165, and VEGF189 are 470, 602, and 674 bp, respectively. Equivalent amounts of expression of these three VEGF isoforms could also be detected by RNase-protection assays. (b) VEGF Western blot and ELISA analysis. Lane 1, parental U87MG cells; lanes 2, 3, and 4, VEGF121-overexpressing clones 7, 12, and 19, respectively; lanes 5, 6, and 7, VEGF165-overexpressing clones 2, 15, and 31, respectively; lanes 8, 9, and 10, VEGF189-overexpressing clones 10, 26, and 30, respectively. For each sample, 30 μg of total cell lysate protein was used. The numbers at the bottom are levels of VEGF secretion determined by ELISA analysis in units of ng per 106 cells. CM was collected after the cells were cultured for 48 hr. (c) VEGF Western blot analysis for heparin-enriched VEGF protein from CM of VEGF165- and VEGF189-overexpressing cells. CM was collected after the cells were cultured for 48 hr in the presence (+) or absence (−) of 100 μg/ml heparin. Designator 1, CM of U87MG cells; designators 2, 3, and 4, CM of VEGF165-overexpressing clones 2, 15, and 31; designators 5, 6, and 7, CM of VEGF189-overexpressing clones 10, 26, and 30. In b and c: , glycosylated VEGF189; •, unglycosylated VEGF189 or glycosylated VEGF165; ▪, unglycosylated VEGF165 or glycosylated VEGF121; and ⧫, unglycosylated VEGF121. Each of the assays was repeated independently three to six times and with cells of various passage numbers, with similar results.
Secreted VEGF121, VEGF165, and VEGF189 Proteins Stimulate Migration of Endothelial Cells.
We next sought to determine whether CM from the parental U87MG and VEGF-overexpressing cells could stimulate the in vitro cell migration of porcine aortic endothelial cells expressing human KDR (PAE/KDR). To reduce the basal activity of the CM from the parental U87MG cells, the volume of CM in each case was adjusted to reflect the final cell numbers of each cell type and then further diluted 100-fold. Table 1 summarizes data generated from the assay using the same set of CM (shown in Fig. 1 b and c) from cells that were treated with heparin for 48 hr. The stimulatory effects of the various CM on PAE/KDR cell migration were directly correlated with their levels of secreted VEGF. In the case of VEGF189, the promotion of cell migration was comparable to that of the other two VEGF isoform proteins when the producing cells were treated with heparin (Table 1). That VEGF was the element in the CM that stimulates PAE/KDR migration was tested by including a VEGF-neutralizing monoclonal antibody in the CM during the migration assay (Table 1). This antibody blocked the stimulation of cell migration elicited by relatively high concentrations (10 ng/ml) of purified rhVEGF165, by CM from the parental U87MG cells, and by the CM from the VEGF121- and VEGF165-overexpressing cells. This neutralizing anti-VEGF antibody did not react with native VEGF189 protein, since it failed to immunoprecipitate it while reacting effectively with VEGF121 and VEGF165 proteins (S.-Y.C., H.-J.S.H., and W.K.C., unpublished data) and so could not be used to test its effects on cell migration in response to VEGF189. The specificity of the inhibition was demonstrated by the absence of similar effects on cell migration by an isotype-matched control monoclonal antibody. The remaining PAE/KDR migration in the CM from the U87MG cells or the CM containing the neutralizing antibody is likely due to other stimulatory substances produced by the tumor cells (18). These data indicate that VEGF was the component in the CM from the VEGF-overexpressing cells that stimulated PAE/KDR migration and that secreted VEGF121, VEGF165 and, likely the VEGF189 proteins in the CM, have similar efficacy for a significant effect of VEGF on endothelial cells in vitro.
Table 1.
Cells overexpressing VEGF have increased abilities to elicit PAE/KDR cell migration
| Medium | No. of migrated cells after addition to
the medium
|
||
|---|---|---|---|
| None | VEGF-neutralizing Ab | Anti-mouse IgG2b.κ Ab | |
| Medium alone | 138.2 ± 5.1 | ND | ND |
| rhVEGF165 | 638.7 ± 26.4 | 144.0 ± 4.9 | 628.2 ± 15.5 |
| CM | |||
| U87MG | 232.2 ± 5.3 | 218.6 ± 0.7 | 236.0 ± 7.0 |
| VEGF121 | 561.0 ± 46.9 | 171.0 ± 6.5 | 542.7 ± 18.5 |
| VEGF165 | 522.6 ± 27.7 | 193.1 ± 2.1 | 499.7 ± 24.5 |
| VEGF189 | |||
| Without heparin | 356.0 ± 8.2 | ND | ND |
| With heparin | 592.3 ± 22.8 | ND | ND |
In vitro migration of PAE/KDR cells in response to purified recombinant human VEGF165 (rhVEGF165, 10 ng/ml; R & D Systems) and to CM was measured in modified Boyden chambers. CM from the parental U87MG or their derivatives was diluted 1:100 with 0.1% BSA/Ham’s F-12 and tested in the absence or presence of either 1 μg/ml neutralizing anti-VEGF antibody or 1 μg/ml IgG2b.κ isotype control monoclonal antibody. Migrated cells were counted in 10 high-powered fields (×400 total magnification) per filter. All samples were examined in triplicate and data are shown as the mean numbers of migrated cells ± SEM. The assay was performed in triplicate for three independent sets of CM at least three times each with similar results. ND, not done.
Implantation of VEGF121- and VEGF165-Overexpressing U87MG Cells into Mouse Brain Causes Intracerebral Hemorrhage.
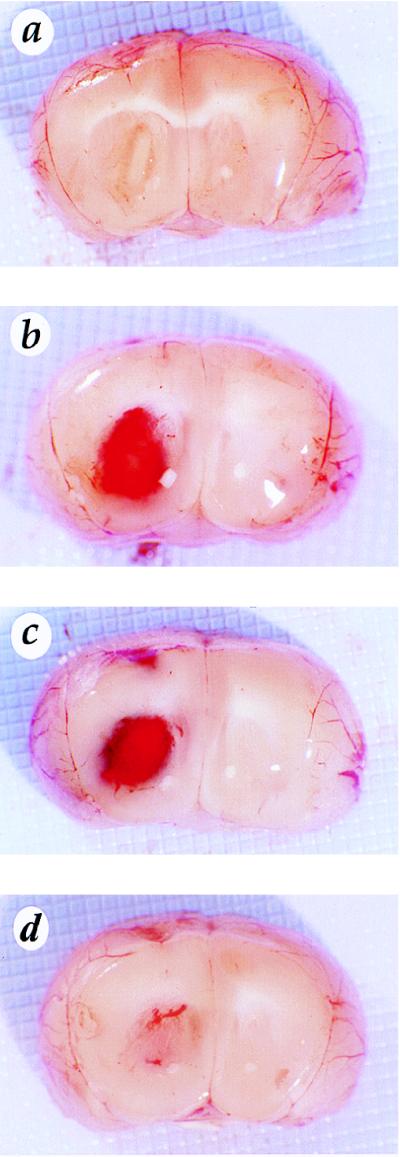
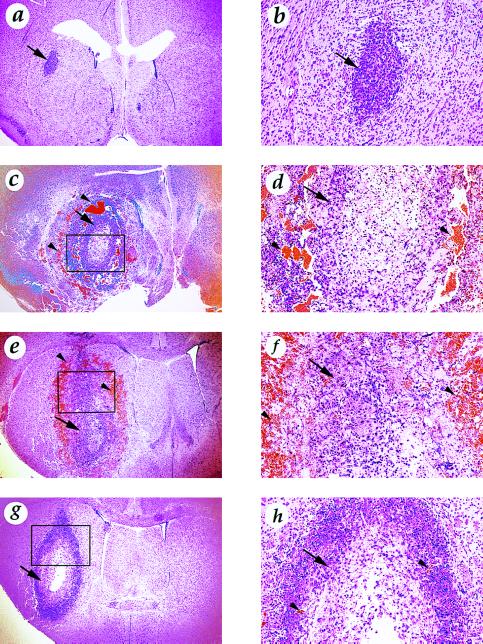
Enhanced in vivo angiogenicity and tumorigenicity have been demonstrated for tumor cells overexpressing VEGF (20, 21). To investigate whether overexpression of each of the three VEGF splice variants in U87MG cells had similar biological effects in vivo, we ectopically implanted the VEGF-overexpressing cells into the brains of nude mice. Comparable numbers of these cell types were implanted: the parental U87MG cells; an equal admixture of cells from VEGF121-overexpressing clones 7, 12, and 19; an equal admixture of cells from VEGF165-overexpressing clones 2, 15, and 31; and an equal admixture of cells from VEGF189-overexpressing clones 10, 26, and 30. Unexpectedly, only 60–90 hr after the implantation, mice receiving VEGF121- or VEGF165-overexpressing cells showed clear symptoms of central nervous system suppression. In contrast, mice inoculated with either VEGF189-overexpressing or parental U87MG cells remained free of such morbidity. Figs. 2 and 3 show examples of the brains of these animals. Each mouse that received VEGF121- and VEGF165-overexpressing cells had readily apparent intracerebral hemorrhage in its brain parenchyma (Fig. 2 b and c). Hematoxylin and eosin staining of these tissues revealed clusters of red blood cells around the tumor (Fig. 3 c, d, e, and f). In contrast, there was no evidence of hemorrhage in the brains of mice implanted with either U87MG parental cells or VEGF189-overexpressing cells, even after more extended growth periods. Rather, the brains of the mice receiving U87MG cells had small tumors, while brains of the mice receiving VEGF189-overexpressing cells contained larger tumors (Fig. 2 a and d and Fig. 3 a, b, g, and h). To confirm this observation of differential effects of these isoforms, the experiments were repeated seven more times each with admixtures of the VEGF121-, VEGF165-, and VEGF189-overexpressing cells; with cells from each of the individual clones; and also with another set of three each VEGF121- and VEGF165-overexpressing clones. In all cases, similar results were obtained (data not shown). Thus, overexpression of the two VEGF splice variants VEGF121 and VEGF165 in U87MG cells caused intracranial hemorrhage in mouse brains implanted with them.
Figure 2.
Overexpression of VEGF121 and VEGF165 but not VEGF189 in U87MG cells causes brain hemorrhage. Brains were implanted with the U87MG cells (a), with VEGF121-overexpressing cells (b), with VEGF165-overexpressing cells (c), or with VEGF189-overexpressing cells (d). (Original magnification, ×10.) The photographs were scanned into computer files and assembled using Adobe Photoshop. Four to six nude mice in each group were implanted intracerebrally, and the mice were sacrificed 60–90 hr later. The experiments were repeated eight times with similar results.
Figure 3.
Hematoxylin and eosin staining of mouse brains that received the parental U87MG or VEGF-overexpressing cells. (a and b) U87MG cells. (c and d) VEGF121-overexpressing cells. (e and f) VEGF165-overexpressing cells. (g and h) VEGF189-overexpressing cells. Original magnifications: a, c, e, and g, ×25; b, d, f, and h, ×100. d, f, and h are the enlarged pictures of the rectangular areas in c, e, and g, respectively. Arrows indicate tumor tissue and arrowheads indicate the blood cells. Experiments were carried out similarly to those shown in Fig. 2.
Development of Hemorrhage Requires Threshold Levels of VEGF Secretion and a Constant Supply.
We next tested whether there was a correspondence between levels of VEGF secretion and intracerebral hemorrhage or whether a threshold level of secretion needed to be attained. The VEGF165-overexpressing clones 2, 15, and 31 were mixed with the parental U87MG cells to form four groups that exhibited VEGF secretion into CM levels of 400, 300, 200, and 100 ng per 106 cells per 48 hr, respectively. These admixtures were implanted into mouse brains, and their abilities to elicit physical symptoms unique to each of the groups of tested mice were determined after 70 hr. Mice injected with the admixture of cells that secreted VEGF at levels of 100 ng showed no symptoms of morbidity. In contrast, mice implanted with the cells that secreted VEGF at levels of 200 ng became lethargic. Those in the third group, which received the cells that secreted VEGF at levels of 300 ng, exhibited a combination of lethargy and myotonic jerking. Mice that were injected with the cells that secreted VEGF at levels of 400 ng became profoundly hemi- or paraparetic. When brains from these four groups of mice were analyzed, punctate bleeding limited to the tumor periphery was observed in the brains of the first group, a coalescing peripheral band of hemorrhage was seen in the brains of the second group, an even thicker peripheral distribution of hemorrhage occurred in the brains of the third group, and large hemorrhages obliterating the appearance of the implanted tumor were apparent in the brains of the fourth group (data not shown). In a separate group of mice, we intracerebrally injected either 500 or 1,000 ng of purified rhVEGF165. These groups showed no morbid symptoms and were sacrificed after 7 days. Notably, there was no change in the vasculature or evidence of angiogenesis in the brains from these groups (data not shown). These experiments suggest that threshold levels of VEGF secretion from VEGF-overexpressing cells and a constant supply of secreted VEGF are two requisites for inducing intracerebral hemorrhage in this system.
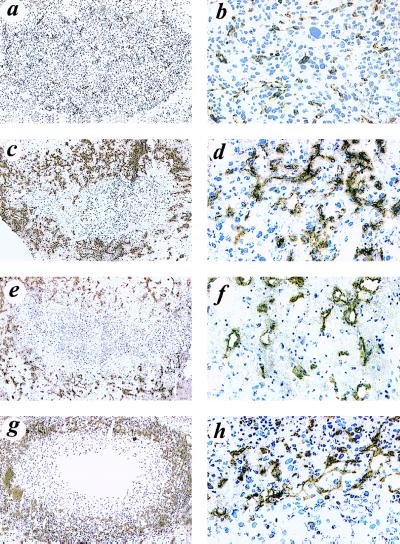
Overexpression of VEGF121 or VEGF165 by U87MG Cells Causes Eruption of Nascent Blood Vessels in Hemorrhagic Mouse Brains.
Immunohistochemical analysis using a polyclonal antibody showed substantial expression of VEGF protein in the expected diffuse pattern in the mouse brains inoculated with VEGF121-, VEGF165-, and VEGF189-overexpressing cells, but showed the expected weaker expression in the brains of animals that received the parental U87MG cells. To analyze the growth and integrity of blood vessels in these brains, we used a monoclonal antibody against the endothelial cell surface protein marker, CD31. As demonstrated in Fig. 4, numerous nascent blood vessels were stained in the areas surrounding the VEGF121-, VEGF165- and VEGF189-overexpressing tumors (Fig. 4 c, d, e, f, g, and h), indicating that overexpression of VEGF promoted blood vessel growth, whereas there was minimal immunoreactivity and vessel growth in the vicinity of the parental U87MG tumors (Fig. 4 a and b). More strikingly, eruption of the nascent blood vessels was observed only in the hemorrhagic brains that received the VEGF121- and VEGF165-overexpressing cells (Fig. 2 b and c). This eruption was further verified by hematoxylin and eosin staining (Fig. 3 c, d, e, and f) and by immunostaining with an anti-von Willebrand factor antibody that reacts with many components of blood (data not shown).
Figure 4.
Nascent blood vessels of intracerebral tumors or hemorrhages formed by parental U87MG cells (a and b), VEGF121-overexpressing cells (c and d), VEGF165-overexpressing cells (e and f), and VEGF189-overexpressing cells (g and h). Immunohistochemistry was performed with a monoclonal anti-CD31 antibody (MEC13; PharMingen) on cryostat mouse brain slices. Original magnifications: a, c, e and g: ×10; b, d, f and H: ×200. The photographs were captured into computer files and contrast-enhanced using Adobe Photoshop. The experiments were carried out as described in the legend of Fig. 3.
Overexpression of VEGF189 by U87MG Cells Increases Their Angiogenicity and Tumorigenicity in Mouse Brain.
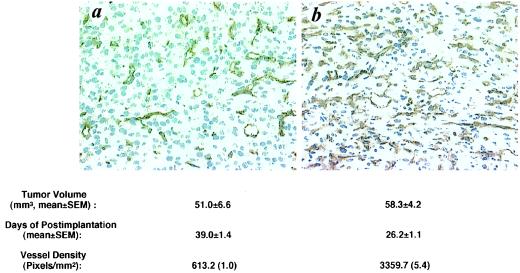
Having demonstrated that overexpression of VEGF189 in U87MG cells did not cause brain hemorrhage, we next sought to determine whether mice that received VEGF189-overexpressing cells were more tumorous than mice implanted with parental U87MG cells. The results are illustrated in Fig. 5 and show that, whereas mice implanted with the parental U87MG cells developed full-size tumors in 39 days, those mice that received the VEGF189-overexpressing cells grew similar-sized tumors in only 26 days. To determine whether the increased levels of VEGF secretion and tumorigenicity were associated with an increase in the ability of the cells to induce neovascularization, we determined the relative blood vessel densities by immunostaining the tumor samples with a monoclonal antibody against CD31. Computerized quantitative image analysis showed that blood vessel densities were 5-fold higher in the tumors formed by the VEGF189-overexpressing cells (Fig. 5b) as compared with those in the tumors formed by the parental U87MG cells (Fig. 5a).
Figure 5.
Overexpression of VEGF189 in U87MG cells enhances angiogenicity and tumorigenicity in mouse brain. (a and b) Immunohistochemical staining with an anti-CD31 mAb. Intracerebral tumors were formed by the parental U87MG cells (a) or the VEGF189-overexpressing cells (b). Original magnification, ×200. The experiments were repeated five times with similar results. Four to six nude mice in each group were implanted intracerebrally and the times for sacrificing the mice are indicated.
DISCUSSION
In addition to being associated with the development of glioblastoma multiforme, hemorrhages are also apparent in infarctions in the brain that often develop from ischemic strokes (22). Because brain ischemia induces increased VEGF expression (23) and increased hemorrhage can occur within 2–3 days after an ischemic stroke, the two events may be mechanistically related. Another instance supporting the association of VEGF expression with hemorrhage comes from von Hippel-Lindau patients, who often develop blindness subsequent to their frequent development of retinal angiomas, which are histologically identical to the hemangioblastomas that occur in the cerebellum and spinal cord. Hemangioblastomas produce a greater amount of VEGF than any other type of tumor of the central nervous system (17) and it is reasonably certain that the retinal angiomas produce VEGF to the same degree. Thus, VEGF expression is correlated with a propensity for hemorrhage in several circumstances.
Here, we show that overexpression of the smaller VEGF isoforms, VEGF121 and VEGF165, causes tumor-associated intracranial hemorrhage in an experimental system. This is, to our knowledge, the first demonstration that intracranial hemorrhage can be caused by eliciting expression of a single growth factor. Immunohistochemical studies indicate that, during the development of such hemorrhages, the brain vasculature experiences rapid growth and breakdown, since numerous nascent blood vessels and blood cells or substances were concentrated around the hemorrhagic tumors, indicating that the vasculature responded vigorously to VEGF (Figs. 2, 3, 4). Our results also show that the development of the intracerebral hemorrhage requires threshold levels of VEGF secretion. This is consistent with the emerging concept that the absolute levels of VEGF are critical to its function in both physiological and pathological conditions. Perhaps most striking in this regard is that in heterozygous knock-out animals, the loss of a single allele of the VEGF gene completely impaired vasculogenesis (24, 25). Moreover, antisense experiments have shown that decreases of VEGF secretion to 15–20% of those of parental tumor cells caused suppression of their angiogenic and tumorigenic capacities in vivo (18, 26). Here, we show that an elevation of VEGF secretion by 4- to 10-fold caused an acute development of tumor-associated hemorrhage. Thus regulation of secreted VEGF levels to either lower or higher than normal levels has serious physiological consequences.
There are similarities and differences between the spliced VEGF variants, VEGF121, VEGF165, and VEGF189, in terms of their biological functions, interactions with extracellular matrix components, and receptor binding (27, 28). Our present studies of VEGF189 overexpression in U87MG cells have several implications for its biological function. First, the level of VEGF189 overexpression was comparable to that of VEGF121 and VEGF165, both within the cells (Fig. 1b) and in the CM (Fig. 1c), whereas the release of VEGF189 protein into CM was enhanced by heparin. Second, the ability of VEGF189 to stimulate endothelial cell migration was also similar to that of VEGF121 and VEGF165 proteins in the CM (Table 1). Third, the VEGF189 protein was biologically functional, as indicated by the increased blood vessel density in the vicinity of VEGF189 tumors compared with the borders of the parental U87MG tumors (Fig. 4 a, b, g, and h), as well as by their enhanced angiogenicity and tumorigenicity (Fig. 5). Fourth, we could not analyze VEGF189 protein secretion by using the VEGF ELISA (Fig. 1b) or neutralize VEGF189 protein stimulating activity for endothelial cell migration with the neutralizing monoclonal anti-VEGF antibody (data not shown). We found that several other anti-VEGF antibodies also did not react with VEGF189 protein. Finally, VEGF121 and VEGF165 are the predominant forms of VEGF expressed in human glioblastomas and VEGF189 is not highly expressed in brain tumors or the normal brain (29, 30). Together, these data may indicate that the VEGF189 protein has a unique secondary conformation and expression pattern in normal and tumor tissues, and might imply a special biological function that has not yet been uncovered.
In summary, by introducing VEGF-overexpressing tumor cells into the brain, we have developed a model that appears to recapitulate the brain tumor-associated hemorrhage phenomenon and that may prove useful for determining its molecular mechanisms. This may be especially so because we can now dissect the rapid multistep process of hemorrhage in the mouse brain by manipulating factors involved in angiogenesis. The differences in function of the three VEGF isoforms in the development of hemorrhage also raise the issue of determining each of their common and divergent physiological roles. Finally, approaches similar to this but that do not employ tumor cells as the continuous source of relevant VEGF isoforms may be useful in investigating intracerebral hemorrhage due to non-cancer-related conditions such as stroke or congenital malformations.
Acknowledgments
We thank Drs. Peter McL. Black, Edward H. Oldfield, and Marsha M. Merrill for their comments on the paper; Xiang-Dong Ji (PharMingen) for preparing cryostat sections of mouse brain samples and critical advice concerning the immunohistochemical analysis; Lena Claesson-Welsh (Ludwig Institute for Cancer Research, Uppsala, Sweden) for the PAE/KDR cells; Degui Wang for advice concerning the endothelial cell migration assay; and Frank Coufal for advice on paraffin tissue sample preparation and helpful discussions throughout the course of the project. S.-Y.C. was a recipient of a Research Training Fellowship from the American Lung Association of California and is supported by National Institutes of Health National Research Service Award HL09391–02. M.N. was an awardee of a fellowship from the Yasuda Medical Research Foundation, Osaka, Japan, and is a fellow of the Japan Brain Foundation.
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
- rhVEGF
recombinant human VEGF
- CM
conditioned medium
- PAE
porcine aortic endothelial cells
References
- 1.Feldmann E. Intracerebral Hemorrhage. Armonk City, NY: Futura; 1994. [Google Scholar]
- 2.Braunwald E, Isselbacher K J, Petersdorf R G, Wilson J D, Martin J B, Fauci A S, editors. Harrison’s Principles of Internal Medicine. 11th Ed. New York: McGraw–Hill; 1987. [Google Scholar]
- 3.Brem S, Cotran R, Folkman J. J Natl Cancer Inst. 1972;48:347–356. [PubMed] [Google Scholar]
- 4.Plate K H, Risau W. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Houck K, Jakeman L, Leung D W. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 6.Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. J Biol Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- 7.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 8.Aiello L P, Avery R L, Arrigg P G, Keyt B A, Jampel H D, Shah S T, Pasquale L R, Thieme H, Iwamoto M A, Park J E, Nguyen H V, Aiello L M, Ferrara N, King G L. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Neurological Disorders and Stroke rt-PA Study Group. N Engl J Med. 1995;333:1581–1587. [Google Scholar]
- 10.Pierce E A, Avery R L, Foley E D, Aiello L P, Smith L E. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 12.Fava R A, Olsen N J, Spencer-Green G, Yeo K T, Yeo T K, Berse B, Jackman R W, Senger D R, Dvorak H F, Brown L F. J Exp Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E. Cardiovasc Res. 1994;28:1176–1179. doi: 10.1093/cvr/28.8.1176. [DOI] [PubMed] [Google Scholar]
- 14.Rothbart D, Awad I A, Lee J, Kim J, Harbaugh R, Criscuolo G R. Neurosurgery. 1996;38:915–924. doi: 10.1097/00006123-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Skirgaudas M, Awad I A, Kim J, Rothbart D, Criscuolo G. Neurosurgery. 1996;39:537–545. doi: 10.1097/00006123-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Kalkanis S N, Carroll R S, Zhang J, Zamani A A, Black P M. J Neurosurg. 1996;85:1095–1101. doi: 10.3171/jns.1996.85.6.1095. [DOI] [PubMed] [Google Scholar]
- 17.Wizigmann-Voos S, Plate K H. Histol Histopathol. 1996;11:1049–1061. [PubMed] [Google Scholar]
- 18.Cheng S Y, Huang H J, Nagane M, Ji X D, Wang D, Shih C C, Arap W, Huang C M, Cavenee W K. Proc Natl Acad Sci USA. 1996;93:8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houck K A, Leung D W, Rowland A M, Winer J, Ferrara N. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 20.Claffey K P, Brown L F, del Aguila L F, Tognazzi K, Yeo K T, Manseau E J, Dvorak H F. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 21.Zhang H T, Craft P, Scott P A, Ziche M, Weich H A, Harris A L, Bicknell R. J Natl Cancer Inst. 1995;87:213–219. doi: 10.1093/jnci/87.3.213. [DOI] [PubMed] [Google Scholar]
- 22.Schehr R S. Nat Biotechnol. 1996;14:1549–1554. doi: 10.1038/nbt1196-1549. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. Stroke. 1996;27:1865–1872. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Nature (London) 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 26.Saleh M, Stacker S A, Wilks A F. Cancer Res. 1996;56:393–401. [PubMed] [Google Scholar]
- 27.Neufeld G, Cohen T, Gitay-Goren H, Poltorak Z, Tessler S, Sharon R, Gengrinovitch S, Levi B Z. Cancer Metastasis Rev. 1996;15:153–158. doi: 10.1007/BF00437467. [DOI] [PubMed] [Google Scholar]
- 28.Takeshita S, Tsurumi Y, Couffinahl T, Asahara T, Bauters C, Symes J, Ferrara N, Isner J M. Lab Invest. 1996;75:487–501. [PubMed] [Google Scholar]
- 29.Berkman R A, Merrill M J, Reinhold W C, Monacci W T, Saxena A, Clark W C, Robertson J T, Ali I U, Oldfield E H. J Clin Invest. 1993;91:153–159. doi: 10.1172/JCI116165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacic M, Edwards N A, Merrill M J. Growth Factors. 1995;12:11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]