Abstract
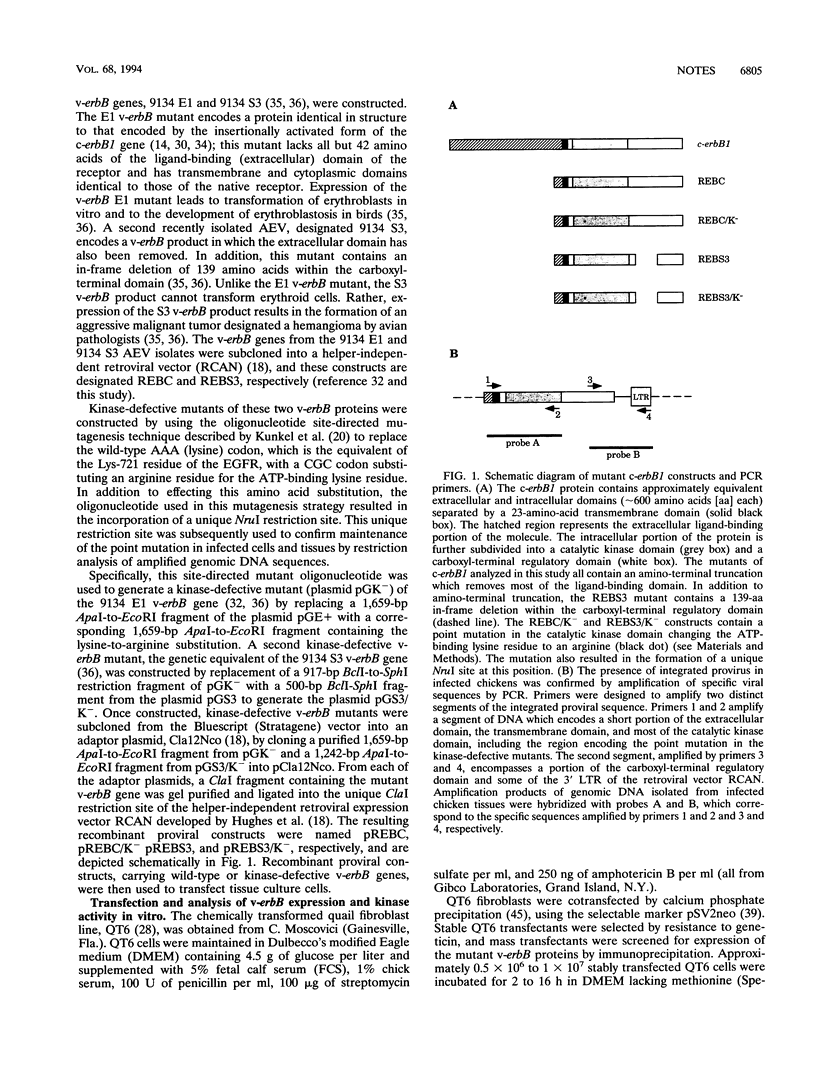
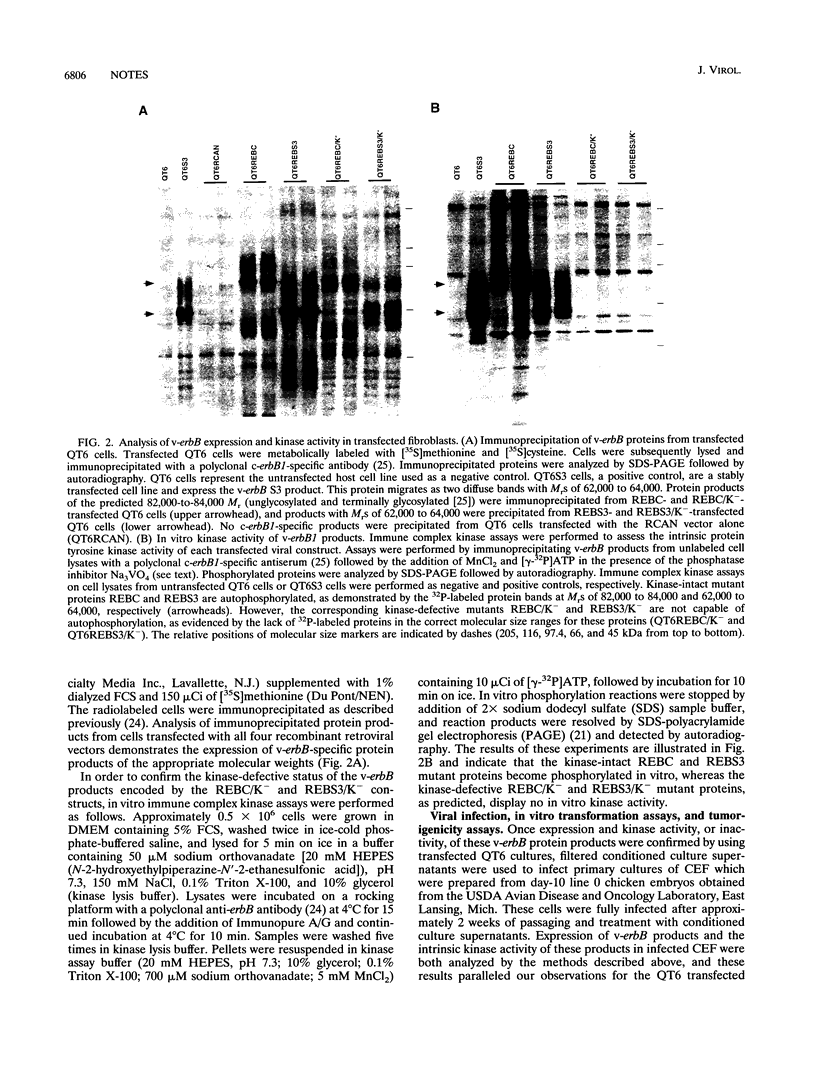
Expression of mutant avian c-erbB1 genes results in tissue-specific transformation in chickens. Site-directed mutagenesis was used to generate kinase-defective mutants of several tissue-specific v-erbB transforming mutants by replacement of the ATP-binding lysine residue in the kinase domain with an arginine residue. These kinase-defective v-erbB mutants were analyzed for their in vitro and in vivo transforming potentials. Specifically, kinase-defective mutants of erythroleukemogenic, hemangioma-inducing, and sarcomagenic v-erbB genes were assessed for their oncogenic potential. In vitro transformation potential was assessed by soft-agar colony formation in primary cultures of chick embryo fibroblasts (CEF). In vivo transformation potential was determined by infection of 1-day-old line 0 chicks with concentrated recombinant retrovirus and then monitoring of birds for tumor formation. These transformation assays demonstrate that kinase activity is absolutely essential for transformation by tissue-specific transforming mutants of the avian c-erbB1 gene. Since all of the tissue-specific v-erbB mutants characterized to date exhibit tyrosine kinase activity in vitro but do not transform all tissues in which they are expressed, we conclude that v-erbB-associated tyrosine kinase activity may be necessary but is not sufficient to induce tumor formation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antczak M., Kung H. J. Transformation of chicken embryo fibroblasts by direct DNA transfection of single oncogenes: comparative analyses of src, erbB, myc, and ras. J Virol. 1990 Apr;64(4):1451–1458. doi: 10.1128/jvi.64.4.1451-1458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Raines M. B., Kung H. J., Vennström B. Rous-associated virus 1-induced erythroleukemic cells exhibit a weakly transformed phenotype in vitro and release c-erbB-containing retroviruses unable to transform fibroblasts. J Virol. 1986 Mar;57(3):1127–1138. doi: 10.1128/jvi.57.3.1127-1138.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-González R., Glenney J. R., Jr Tyrosine phosphorylation of mitogen-activated protein kinase in cells with tyrosine kinase-negative epidermal growth factor receptors. J Biol Chem. 1992 Jul 25;267(21):14535–14538. [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Coker K. J., Staros J. V., Guyer C. A. A kinase-negative epidermal growth factor receptor that retains the capacity to stimulate DNA synthesis. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6967–6971. doi: 10.1073/pnas.91.15.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly P. A., Stern D. F. The epidermal growth factor receptor and the product of the neu protooncogene are members of a receptor tyrosine phosphorylation cascade. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6054–6057. doi: 10.1073/pnas.87.16.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Roussel M. F., Sherr C. J. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol Cell Biol. 1989 Jul;9(7):2890–2896. doi: 10.1128/mcb.9.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand A. J., Sugawa N., James C. D., Collins V. P. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Gamett D. C., Tracy S. E., Robinson H. L. Differences in sequences encoding the carboxyl-terminal domain of the epidermal growth factor receptor correlate with differences in the disease potential of viral erbB genes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6053–6057. doi: 10.1073/pnas.83.16.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkemeyer M., West S. R., Gertler F. B., Hoffmann F. M. A novel tyrosine kinase-independent function of Drosophila abl correlates with proper subcellular localization. Cell. 1990 Nov 30;63(5):949–960. doi: 10.1016/0092-8674(90)90498-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Faria T. N., Stannard B., Roberts C. T., Jr, LeRoith D. Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (alpha IR-3). J Biol Chem. 1993 Feb 5;268(4):2655–2661. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lax I., Johnson A., Howk R., Sap J., Bellot F., Winkler M., Ullrich A., Vennstrom B., Schlessinger J., Givol D. Chicken epidermal growth factor (EGF) receptor: cDNA cloning, expression in mouse cells, and differential binding of EGF and transforming growth factor alpha. Mol Cell Biol. 1988 May;8(5):1970–1978. doi: 10.1128/mcb.8.5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihle N. J., Kung H. J. C-erbB and the epidermal growth-factor receptor: a molecule with dual identity. Biochim Biophys Acta. 1989 Feb;948(3):287–304. doi: 10.1016/0304-419x(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Maihle N. J., Raines M. A., Flickinger T. W., Kung H. J. Proviral insertional activation of c-erbB: differential processing of the protein products arising from two alternate transcripts. Mol Cell Biol. 1988 Nov;8(11):4868–4876. doi: 10.1128/mcb.8.11.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malden L. T., Novak U., Kaye A. H., Burgess A. W. Selective amplification of the cytoplasmic domain of the epidermal growth factor receptor gene in glioblastoma multiforme. Cancer Res. 1988 May 15;48(10):2711–2714. [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Moller D. E., Benecke H., Flier J. S. Biologic activities of naturally occurring human insulin receptor mutations. Evidence that metabolic effects of insulin can be mediated by a kinase-deficient insulin receptor mutant. J Biol Chem. 1991 Jun 15;266(17):10995–11001. [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Nair N., Davis R. J., Robinson H. L. Protein tyrosine kinase activities of the epidermal growth factor receptor and ErbB proteins: correlation of oncogenic activation with altered kinetics. Mol Cell Biol. 1992 May;12(5):2010–2016. doi: 10.1128/mcb.12.5.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Pelley R. J., Maihle N. J., Boerkoel C., Shu H. K., Carter T. H., Moscovici C., Kung H. J. Disease tropism of c-erbB: effects of carboxyl-terminal tyrosine and internal mutations on tissue-specific transformation. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7164–7168. doi: 10.1073/pnas.86.18.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelley R. J., Moscovici C., Hughes S., Kung H. J. Proviral-activated c-erbB is leukemogenic but not sarcomagenic: characterization of a replication-competent retrovirus containing the activated c-erbB. J Virol. 1988 May;62(5):1840–1844. doi: 10.1128/jvi.62.5.1840-1844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent S. A., Lemoine N. R. The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res. 1992;4(1):1–24. doi: 10.1016/0955-2235(92)90002-y. [DOI] [PubMed] [Google Scholar]
- Raines M. A., Lewis W. G., Crittenden L. B., Kung H. J. c-erbB activation in avian leukosis virus-induced erythroblastosis: clustered integration sites and the arrangement of provirus in the c-erbB alleles. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2287–2291. doi: 10.1073/pnas.82.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Maihle N. J., Moscovici C., Crittenden L., Kung H. J. Mechanism of c-erbB transduction: newly released transducing viruses retain poly(A) tracts of erbB transcripts and encode C-terminally intact erbB proteins. J Virol. 1988 Jul;62(7):2437–2443. doi: 10.1128/jvi.62.7.2437-2443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Maihle N. J., Moscovici C., Moscovici M. G., Kung H. J. Molecular characterization of three erbB transducing viruses generated during avian leukosis virus-induced erythroleukemia: extensive internal deletion near the kinase domain activates the fibrosarcoma- and hemangioma-inducing potentials of erbB. J Virol. 1988 Jul;62(7):2444–2452. doi: 10.1128/jvi.62.7.2444-2452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva E., Raden D. L., Davis R. J. Mitogen-activated protein kinase stimulation by a tyrosine kinase-negative epidermal growth factor receptor. J Biol Chem. 1993 Jan 25;268(3):2250–2254. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sung C. K., Maddux B. A., Hawley D. M., Goldfine I. D. Monoclonal antibodies mimic insulin activation of ribosomal protein S6 kinase without activation of insulin receptor tyrosine kinase. Studies in cells transfected with normal and mutant human insulin receptors. J Biol Chem. 1989 Nov 15;264(32):18951–18959. [PubMed] [Google Scholar]
- Taglienti-Sian C. A., Banner B., Davis R. J., Robinson H. L. Induction of renal adenocarcinoma by a nonmutated erbB oncogene. J Virol. 1993 Feb;67(2):1132–1136. doi: 10.1128/jvi.67.2.1132-1136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S. E., Woda B. A., Robinson H. L. Induction of angiosarcoma by a c-erbB transducing virus. J Virol. 1985 May;54(2):304–310. doi: 10.1128/jvi.54.2.304-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Weiner D. B., Kokai Y., Wada T., Cohen J. A., Williams W. V., Greene M. I. Linkage of tyrosine kinase activity with transforming ability of the p185neu oncoprotein. Oncogene. 1989 Oct;4(10):1175–1183. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wong A. J., Ruppert J. M., Bigner S. H., Grzeschik C. H., Humphrey P. A., Bigner D. S., Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Littman D. R. A kinase-independent function of Lck in potentiating antigen-specific T cell activation. Cell. 1993 Aug 27;74(4):633–643. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Ohba Y., Tamaoki N., Shibuya M. A deletion mutation within the ligand binding domain is responsible for activation of epidermal growth factor receptor gene in human brain tumors. Jpn J Cancer Res. 1990 Aug;81(8):773–779. doi: 10.1111/j.1349-7006.1990.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]