Abstract
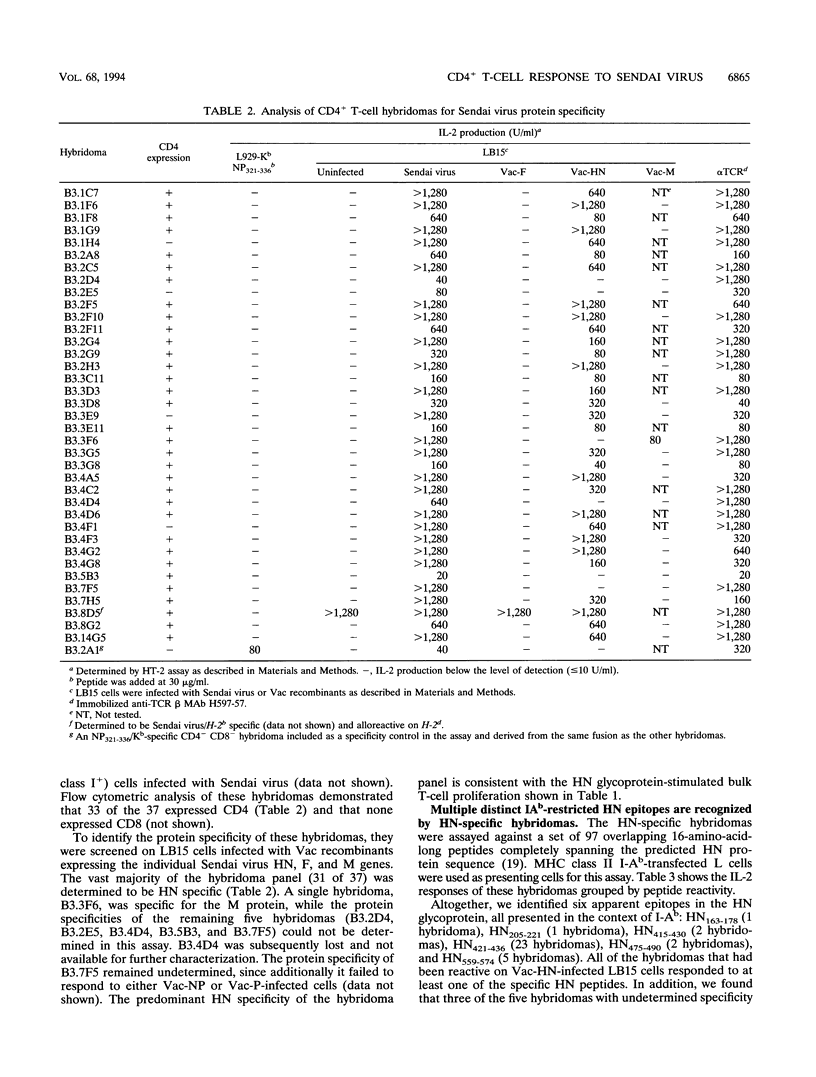
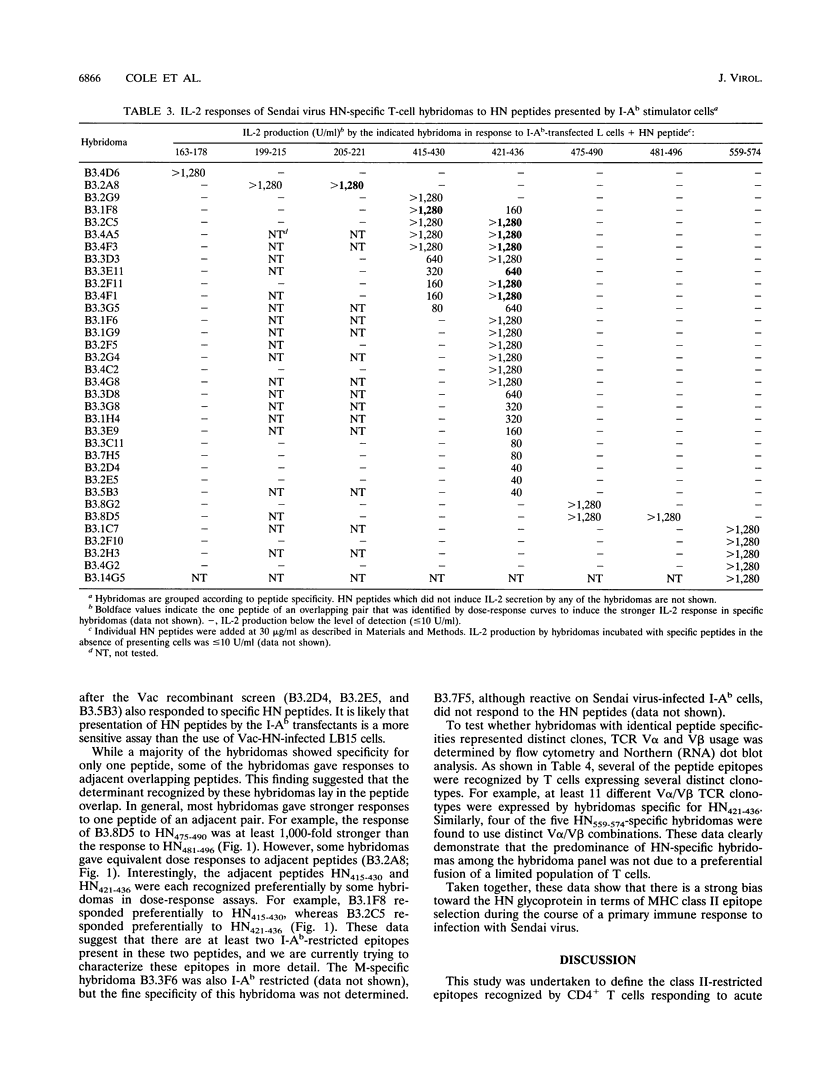
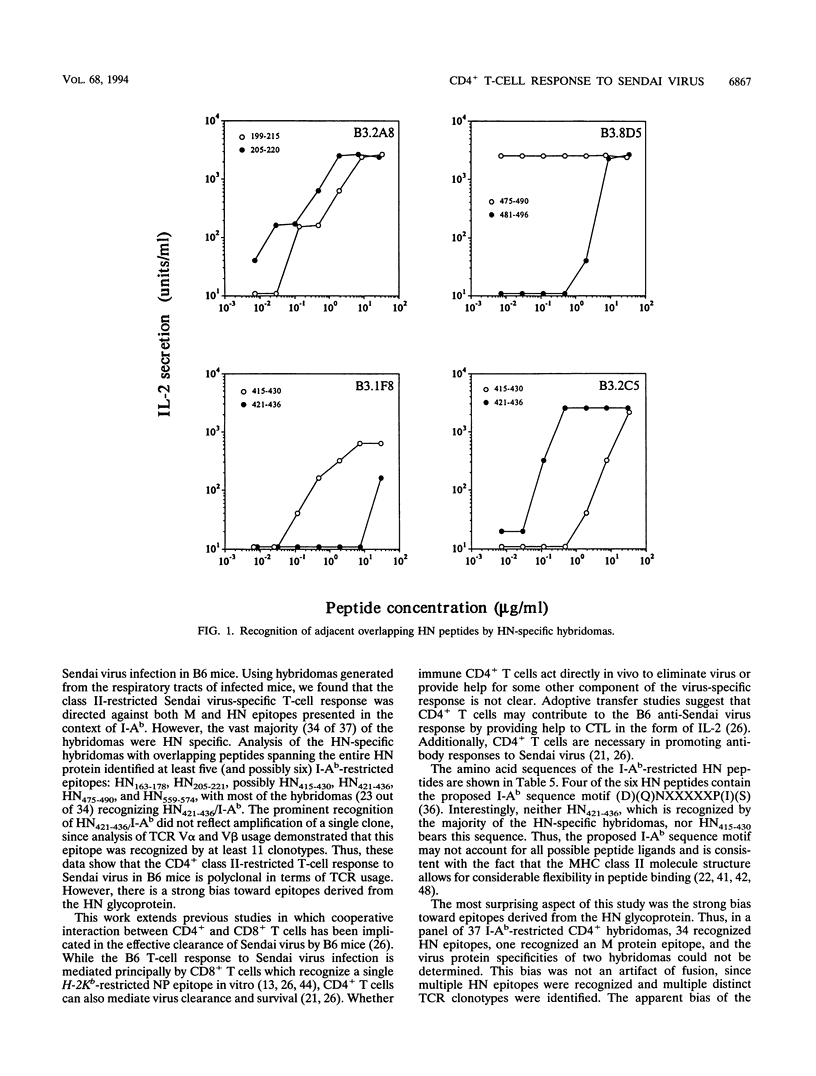
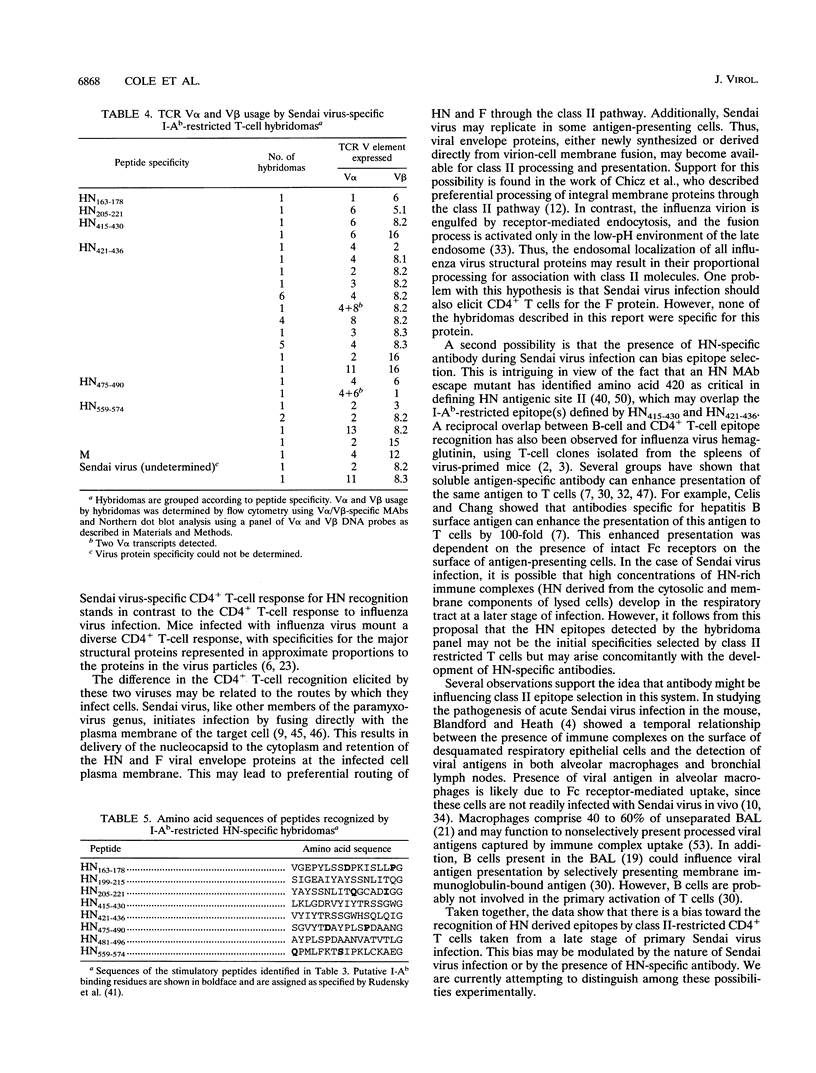
Sendai virus infection of C57BL/6 mice elicits a strong CD4+ and CD8+ T-cell response in the respiratory tract. To investigate the specificity of the CD4+ T-cell response, a panel of hybridomas was generated from cells recovered from the respiratory tracts of infected mice. Using vaccinia virus recombinants expressing individual Sendai virus proteins, we found that the majority of these hybridomas (34 of 37) were specific for the hemagglutinin-neuraminidase (HN) glycoprotein. The hybridomas were then analyzed for reactivity to a set of overlapping peptides spanning the entire length of the hemagglutinin-neuraminidase glycoprotein. At least five H-2 I-Ab-restricted epitopes were defined in HN. The strong bias toward recognition of class II epitopes derived from a single viral protein contrasts with T-cell recognition of epitopes of several proteins in influenza A virus as found previously by others.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan W., Tabi Z., Cleary A., Doherty P. C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990 May 15;144(10):3980–3986. [PubMed] [Google Scholar]
- Barnett B. C., Burt D. S., Graham C. M., Warren A. P., Skehel J. J., Thomas D. B. I-Ad restricted T cell recognition of influenza hemagglutinin. Synthetic peptides identify multiple epitopes corresponding to antibody-binding regions of the HA1 subunit. J Immunol. 1989 Oct 15;143(8):2663–2669. [PubMed] [Google Scholar]
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
- Blandford G., Heath R. B. Studies on the immune response and pathogenesis of Sendai virus infection of mice. I. The fate of viral antigens. Immunology. 1972 Apr;22(4):637–649. [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J., Braciale V. L. Antigen presentation: structural themes and functional variations. Immunol Today. 1991 Apr;12(4):124–129. doi: 10.1016/0167-5699(91)90096-C. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Gerhard W. The diversity of the CD4+ T cell response in influenza. Semin Immunol. 1992 Apr;4(2):85–90. [PubMed] [Google Scholar]
- Celis E., Chang T. W. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science. 1984 Apr 20;224(4646):297–299. doi: 10.1126/science.6231724. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton D., Blandford G. Immunoglobulin class-specific antibody response in serum, spleen, lungs, and bronchoalveolar washings after primary and secondary sendai virus infection of germfree mice. Infect Immun. 1977 Sep;17(3):521–527. doi: 10.1128/iai.17.3.521-527.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Gorga J. C., Vignali D. A., Lane W. S., Strominger J. L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993 Jul 1;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckhut A. M., Allan W., McMickle A., Eichelberger M., Blackman M. A., Doherty P. C., Woodland D. L. Prominent usage of V beta 8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J Immunol. 1993 Sep 1;151(5):2658–2666. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Eichelberger M., Carding S. R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Endres R. O., Marrack P., Kappler J. W. An IL 2-secreting T cell hybridoma that responds to a self class I histocompatibility antigen in the H-2D region. J Immunol. 1983 Oct;131(4):1656–1662. [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Gorman W. L., Gill D. S., Scroggs R. A., Portner A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and Sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology. 1990 Mar;175(1):211–221. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- Heilman C. A. From the National Institute of Allergy and Infectious Diseases and the World Health Organization. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990 Mar;161(3):402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- Hou S., Doherty P. C., Zijlstra M., Jaenisch R., Katz J. M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992 Aug 15;149(4):1319–1325. [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Hackett C. J., McAndrew E. C., Gerhard W. Murine TH response to influenza virus: recognition of hemagglutinin, neuraminidase, matrix, and nucleoproteins. J Immunol. 1985 Mar;134(3):1994–1998. [PubMed] [Google Scholar]
- Iwai H., Machii K., Otsuka Y., Ueda K. T cells subsets responsible for clearance of Sendai virus from infected mouse lungs. Microbiol Immunol. 1988;32(3):305–315. doi: 10.1111/j.1348-0421.1988.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Iwai H., Yamamoto S., Otsuka Y., Ueda K. Cooperation between humoral factor(s) and Lyt-2+ T cells in effective clearance of Sendai virus from infected mouse lungs. Microbiol Immunol. 1989;33(11):915–927. doi: 10.1111/j.1348-0421.1989.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Bronkhorst A. M., de Waal L. P., Melief C. J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986 Sep 1;164(3):723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- Leclercq L., Bismuth G., Thèze J. Antigen-specific helper T-cell clone supernatant is sufficient to induce both polyclonal proliferation and differentiation of small resting B lymphocytes. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6491–6495. doi: 10.1073/pnas.81.20.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca F., Fenoglio D., Kunkl A., Cambiaggi C., Sasso M., Celada F. Differential activation of T cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J Immunol. 1988 May 1;140(9):2893–2898. [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Murphy F. A. Parainfluenza virus Sendai infection in macrophages, ependyma, choroid plexus, vascular endothelium and respiratory tract of mice. Am J Pathol. 1973 Mar;70(3):315–328. [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Ploegh H. L. Intracellular transport of MHC class II molecules. Immunol Today. 1992 May;13(5):179–184. doi: 10.1016/0167-5699(92)90123-O. [DOI] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Marx P. A., Kingsbury D. W. Isolation and characterization of Sendai virus temperature-sensitive mutants. J Virol. 1974 Feb;13(2):298–304. doi: 10.1128/jvi.13.2.298-304.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Metzger D. W. Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology. 1987 May;158(1):61–68. doi: 10.1016/0042-6822(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., al-Ramadi B. K., Rothbard J., Janeway C. A., Jr Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature. 1992 Oct 1;359(6394):429–431. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T., Takao S., Kiyotani K., Fujii Y., Nakayama T., Yoshida T. Expression of the HN, F, NP and M proteins of Sendai virus by recombinant vaccinia viruses and their contribution to protective immunity against Sendai virus infections in mice. J Gen Virol. 1993 Mar;74(Pt 3):479–484. doi: 10.1099/0022-1317-74-3-479. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schumacher T. N., De Bruijn M. L., Vernie L. N., Kast W. M., Melief C. J., Neefjes J. J., Ploegh H. L. Peptide selection by MHC class I molecules. Nature. 1991 Apr 25;350(6320):703–706. doi: 10.1038/350703a0. [DOI] [PubMed] [Google Scholar]
- Squire C. M., Studer E. J., Lees A., Finkelman F. D., Conrad D. H. Antigen presentation is enhanced by targeting antigen to the Fc epsilon RII by antigen-anti-Fc epsilon RII conjugates. J Immunol. 1994 May 1;152(9):4388–4396. [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. D., Laver W. G., Murti K. G., Portner A. Isolation of a biologically active soluble form of the hemagglutinin-neuraminidase protein of Sendai virus. J Virol. 1988 Dec;62(12):4653–4660. doi: 10.1128/jvi.62.12.4653-4660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. D., Portner A. Localization of functional sites on the hemagglutinin-neuraminidase glycoprotein of Sendai virus by sequence analysis of antigenic and temperature-sensitive mutants. Virology. 1987 Sep;160(1):1–8. doi: 10.1016/0042-6822(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Woodland D., Happ M. P., Bill J., Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990 Feb 23;247(4945):964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]



