Abstract
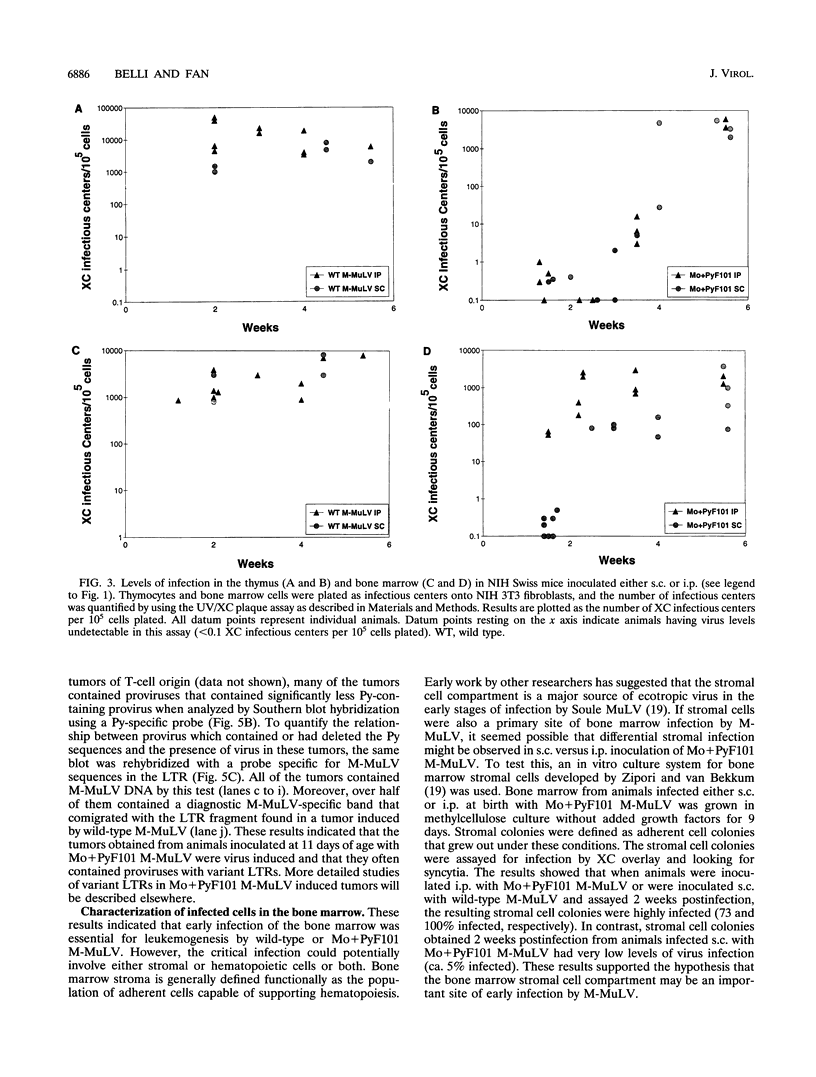
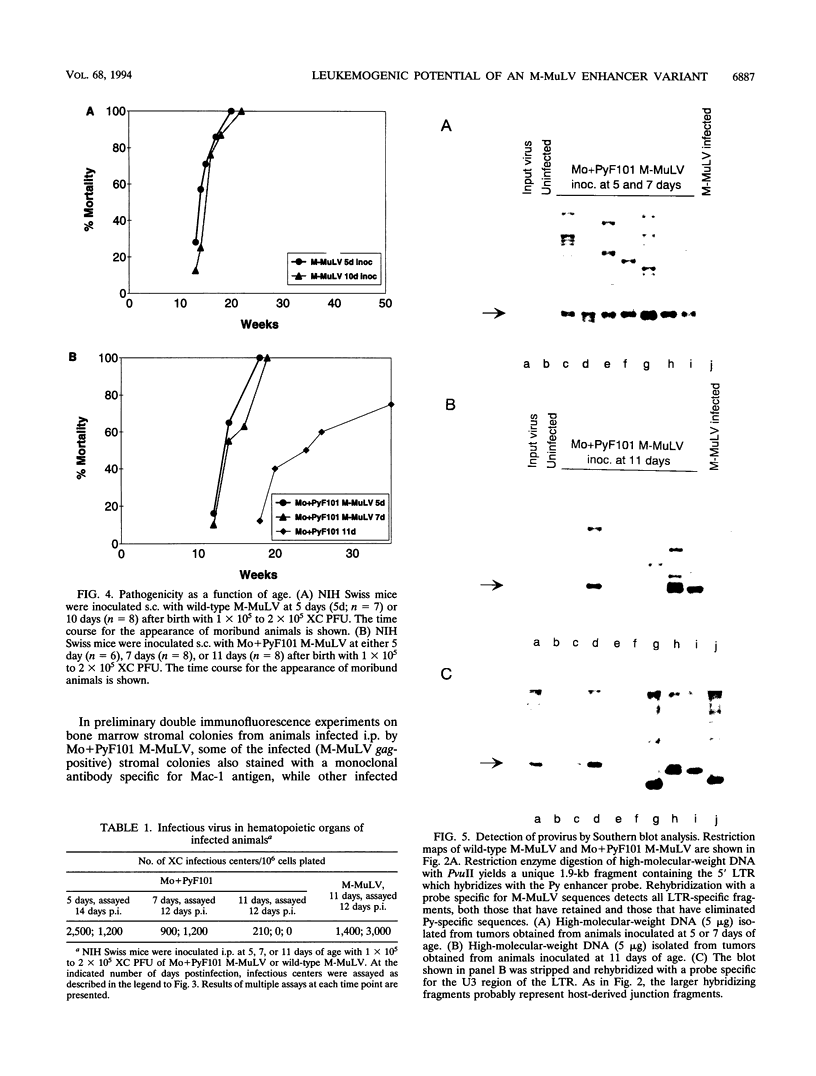
We previously showed that the Mo+PyF101 variant of Moloney murine leukemia virus (M-MuLV) is poorly leukemogenic when inoculated subcutaneously (s.c.) into neonatal mice. We recently found that intraperitoneal (i.p.) inoculation of neonatal mice with the same virus significantly enhanced its leukemogenicity. In this study, infections of neonatal mice by the two different routes of inoculation were compared. We studied replication of the virus in vivo to identify critical preleukemic events. These would be observed in mice inoculated i.p. by Mo+PyF101 M-MuLV but not when inoculation was s.c. Infectious center assays indicated that regardless of the route of inoculation, Mo+PyF101 M-MuLV showed delayed infection of the thymus compared with wild-type M-MuLV. On the other hand, i.p.-inoculated mice showed more rapid appearance of infectious centers in the bone marrow than did s.c.-inoculated animals. Thus, the enhanced leukemogenicity of i.p. inoculation correlated with efficient early infection of the bone marrow and not with early infection of the thymus. These results suggest a role for bone marrow infection for efficient leukemogenesis in Mo+PyF101 M-MuLV-infected mice. Consistent with this notion, if bone marrow infection was decreased by injecting 10- to 12-day-old animals i.p., leukemogenicity resembled that of s.c. inoculation. Thus, two cell types that are critical for the induction of efficient leukemia were implicated. One cell delivers virus from the site of s.c. inoculation (the skin) to the bone marrow and is apparently restricted for Mo+PyF101 M-MuLV replication. The second cell is in the bone marrow, and its early infection is required for efficient leukemogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brightman B. K., Rein A., Trepp D. J., Fan H. An enhancer variant of Moloney murine leukemia virus defective in leukemogenesis does not generate detectable mink cell focus-inducing virus in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2264–2268. doi: 10.1073/pnas.88.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckheit R. W., Jr, Bolognesi D. P., Weinhold K. J. The effects of leukemosuppressive immunotherapy on bone marrow infectious cell centers in AKR mice. Virology. 1987 Apr;157(2):387–396. doi: 10.1016/0042-6822(87)90281-9. [DOI] [PubMed] [Google Scholar]
- Buckheit R. W., Jr, Bolognesi D. P., Weinhold K. J. The role of bone marrow and thymic elements in the initiation and spread of virus production in the AKR thymus. Virology. 1988 Oct;166(2):533–541. doi: 10.1016/0042-6822(88)90524-7. [DOI] [PubMed] [Google Scholar]
- Buckheit R. W., Jr, Kurtzberg J., Bolognesi D. P., Weinhold K. J. The coenrichment of stem cells, prothymocytes, and stromal elements with ecotropic retrovirus-producing cells from the bone marrow of leukemia-prone AKR mice. Virology. 1988 Feb;162(2):354–361. doi: 10.1016/0042-6822(88)90475-8. [DOI] [PubMed] [Google Scholar]
- Davis B. R., Chandy K. G., Brightman B. K., Gupta S., Fan H. Effects of nonleukemogenic and wild-type Moloney murine leukemia virus on lymphoid cells in vivo: identification of a preleukemic shift in thymocyte subpopulations. J Virol. 1986 Nov;60(2):423–430. doi: 10.1128/jvi.60.2.423-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Linney E., Fan H. Suppression of leukaemia virus pathogenicity by polyoma virus enhancers. Nature. 1985 Apr 11;314(6011):550–553. doi: 10.1038/314550a0. [DOI] [PubMed] [Google Scholar]
- Fan H., Chute H., Chao E., Pattengale P. K. Leukemogenicity of Moloney murine leukemia viruses carrying polyoma enhancer sequences in the long terminal repeat is dependent on the nature of the inserted polyoma sequences. Virology. 1988 Sep;166(1):58–65. doi: 10.1016/0042-6822(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Fan H., Mittal S., Chute H., Chao E., Pattengale P. K. Rearrangements and insertions in the Moloney murine leukemia virus long terminal repeat alter biological properties in vivo and in vitro. J Virol. 1986 Oct;60(1):204–214. doi: 10.1128/jvi.60.1.204-214.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Pattengale P. K., Fan H. Addition of substitution of simian virus 40 enhancer sequences into the Moloney murine leukemia virus (M-MuLV) long terminal repeat yields infectious M-MuLV with altered biological properties. J Virol. 1988 Jul;62(7):2427–2436. doi: 10.1128/jvi.62.7.2427-2436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. X., Fan H. Bone marrow depletion by 89Sr complements a preleukemic defect in a long terminal repeat variant of Moloney murine leukemia virus. J Virol. 1991 Aug;65(8):4442–4448. doi: 10.1128/jvi.65.8.4442-4448.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. X., Fan H. Combined infection by Moloney murine leukemia virus and a mink cell focus-forming virus recombinant induces cytopathic effects in fibroblasts or in long-term bone marrow cultures from preleukemic mice. J Virol. 1990 Aug;64(8):3701–3711. doi: 10.1128/jvi.64.8.3701-3711.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rubin D. H., Fields B. N. Molecular basis of reovirus virulence. Role of the M2 gene. J Exp Med. 1980 Oct 1;152(4):853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch T. G., Arnstein P., Manohar V., Leiserson W. M., Chused T. M. Proliferation of infected lymphoid precursors before Moloney murine leukemia virus-induced T-cell lymphoma. J Natl Cancer Inst. 1985 Jan;74(1):137–143. [PubMed] [Google Scholar]
- Zipori D., van Bekkum D. W. Changes in the fibroblastoid colony forming unit population from mouse bone marrow in early stages of Soule virus induced murine leukemia. Exp Hematol. 1979 Mar;7(3):137–144. [PubMed] [Google Scholar]