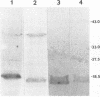
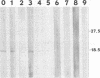
Abstract
Borna disease virus is a nonsegmented negative-strand RNA virus that causes neurologic disease in a wide variety of animal hosts. Here we describe identification and characterization of the first glycoprotein in this viral system. The 18-kDa glycoprotein, gp18, has been purified from infected rat brain. Isolation and microsequencing of this protein allowed identification of a 16.2-kDa open reading frame in the viral antigenome. Lectin binding and endoglycosidase sensitivity assays indicate that gp18 is an unusual N-linked glycoprotein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Niemann H., Smeekens S., Rottier P., Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984 Apr 19;308(5961):751–752. doi: 10.1038/308751a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E., Legler G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem J. 1981 Jun 1;195(3):639–644. doi: 10.1042/bj1950639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L., Ferszt R., Czech G. Borna disease virus infection and affective disorders in man. Arch Virol Suppl. 1993;7:159–167. doi: 10.1007/978-3-7091-9300-6_13. [DOI] [PubMed] [Google Scholar]
- Bode L., Riegel S., Ludwig H., Amsterdam J. D., Lange W., Koprowski H. Borna disease virus-specific antibodies in patients with HIV infection and with mental disorders. Lancet. 1988 Sep 17;2(8612):689–689. doi: 10.1016/s0140-6736(88)90505-3. [DOI] [PubMed] [Google Scholar]
- Briese T., Schneemann A., Lewis A. J., Park Y. S., Kim S., Ludwig H., Lipkin W. I. Genomic organization of Borna disease virus. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T., de la Torre J. C., Lewis A., Ludwig H., Lipkin W. I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Duchala C. S., Griffin J. W., Kincaid A. L., Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987 Nov;61(11):3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Moench T. R., Lipkin W. I. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991 May;50(3):205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- Carbone K. M., Rubin S. A., Sierra-Honigmann A. M., Lederman H. M. Characterization of a glial cell line persistently infected with borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993 Mar;67(3):1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cubitt B., Oldstone C., de la Torre J. C. Sequence and genome organization of Borna disease virus. J Virol. 1994 Mar;68(3):1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Goldstein I. J. Bandeiraea simplicifolia lectin II. Methods Enzymol. 1978;50:350–354. doi: 10.1016/0076-6879(78)50041-4. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Karhi K. K., Kämpe O., Peterson P. A., Andersson L. C. Glycophorin A: in vitro biogenesis and processing. Methods Enzymol. 1983;96:281–298. doi: 10.1016/s0076-6879(83)96026-3. [DOI] [PubMed] [Google Scholar]
- Hayes B. K., Varki A. The biosynthesis of oligosaccharides in intact Golgi preparations from rat liver. Analysis of N-linked and O-linked glycans labeled by UDP-[6-3H]N-acetylgalactosamine. J Biol Chem. 1993 Aug 5;268(22):16170–16178. [PubMed] [Google Scholar]
- Herzog S., Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168(3):153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lau J. T., Welply J. K., Shenbagamurthi P., Naider F., Lennarz W. J. Substrate recognition by oligosaccharyl transferase. Inhibition of co-translational glycosylation by acceptor peptides. J Biol Chem. 1983 Dec 25;258(24):15255–15260. [PubMed] [Google Scholar]
- Lipkin W. I., Travis G. H., Carbone K. M., Wilson M. C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H., Bode L., Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- Lundgren A. L., Czech G., Bode L., Ludwig H. Natural Borna disease in domestic animals others than horses and sheep. Zentralbl Veterinarmed B. 1993 Jun;40(4):298–303. doi: 10.1111/j.1439-0450.1993.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Malkinson M., Weisman Y., Ashash E., Bode L., Ludwig H. Borna disease in ostriches. Vet Rec. 1993 Sep 18;133(12):304–304. doi: 10.1136/vr.133.12.304-b. [DOI] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Thibault K. J., Hatalski C. G., Lipkin W. I. Sequence similarity between Borna disease virus p40 and a duplicated domain within the paramyxovirus and rhabdovirus polymerase proteins. J Virol. 1992 Nov;66(11):6572–6577. doi: 10.1128/jvi.66.11.6572-6577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna virus in the brain. Science. 1983 Jun 24;220(4604):1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- Ogata S., Muramatsu T., Kobata A. Fractionation of glycopeptides by affinity column chromatography on concanavalin A-sepharose. J Biochem. 1975 Oct;78(4):687–696. doi: 10.1093/oxfordjournals.jbchem.a130956. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Pyper J. M., Richt J. A., Brown L., Rott R., Narayan O., Clements J. E. Genomic organization of the structural proteins of borna disease virus revealed by a cDNA clone encoding the 38-kDa protein. Virology. 1993 Jul;195(1):229–238. doi: 10.1006/viro.1993.1364. [DOI] [PubMed] [Google Scholar]
- Rott R., Herzog S., Fleischer B., Winokur A., Amsterdam J., Dyson W., Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985 May 10;228(4700):755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- Schneider P. A., Briese T., Zimmermann W., Ludwig H., Lipkin W. I. Sequence conservation in field and experimental isolates of Borna disease virus. J Virol. 1994 Jan;68(1):63–68. doi: 10.1128/jvi.68.1.63-68.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schädler R., Diringer H., Ludwig H. Isolation and characterization of a 14500 molecular weight protein from brains and tissue cultures persistently infected with borna disease virus. J Gen Virol. 1985 Nov;66(Pt 11):2479–2484. doi: 10.1099/0022-1317-66-11-2479. [DOI] [PubMed] [Google Scholar]
- Scudder P., Hanfland P., Uemura K., Feizi T. Endo-beta-D-galactosidases of Bacteroides fragilis and Escherichia freundii hydrolyze linear but not branched oligosaccharide domains of glycolipids of the neolacto series. J Biol Chem. 1984 May 25;259(10):6586–6592. [PubMed] [Google Scholar]
- Shainkin R., Perlmann G. E. Phosvitin, a phosphoglycoprotein. I. Isolation and characterization of a glycopeptide from phosvitin. J Biol Chem. 1971 Apr 10;246(7):2278–2284. [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- Thiedemann N., Presek P., Rott R., Stitz L. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J Gen Virol. 1992 May;73(Pt 5):1057–1064. doi: 10.1099/0022-1317-73-5-1057. [DOI] [PubMed] [Google Scholar]
- Thierer J., Riehle H., Grebenstein O., Binz T., Herzog S., Thiedemann N., Stitz L., Rott R., Lottspeich F., Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992 Feb;73(Pt 2):413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- VandeWoude S., Richt J. A., Zink M. C., Rott R., Narayan O., Clements J. E. A borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990 Nov 30;250(4985):1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Carbone K. M., Lipkin W. I. Molecular characterization of the Borna disease agent. Virology. 1990 Dec;179(2):853–856. doi: 10.1016/0042-6822(90)90154-j. [DOI] [PubMed] [Google Scholar]