Abstract
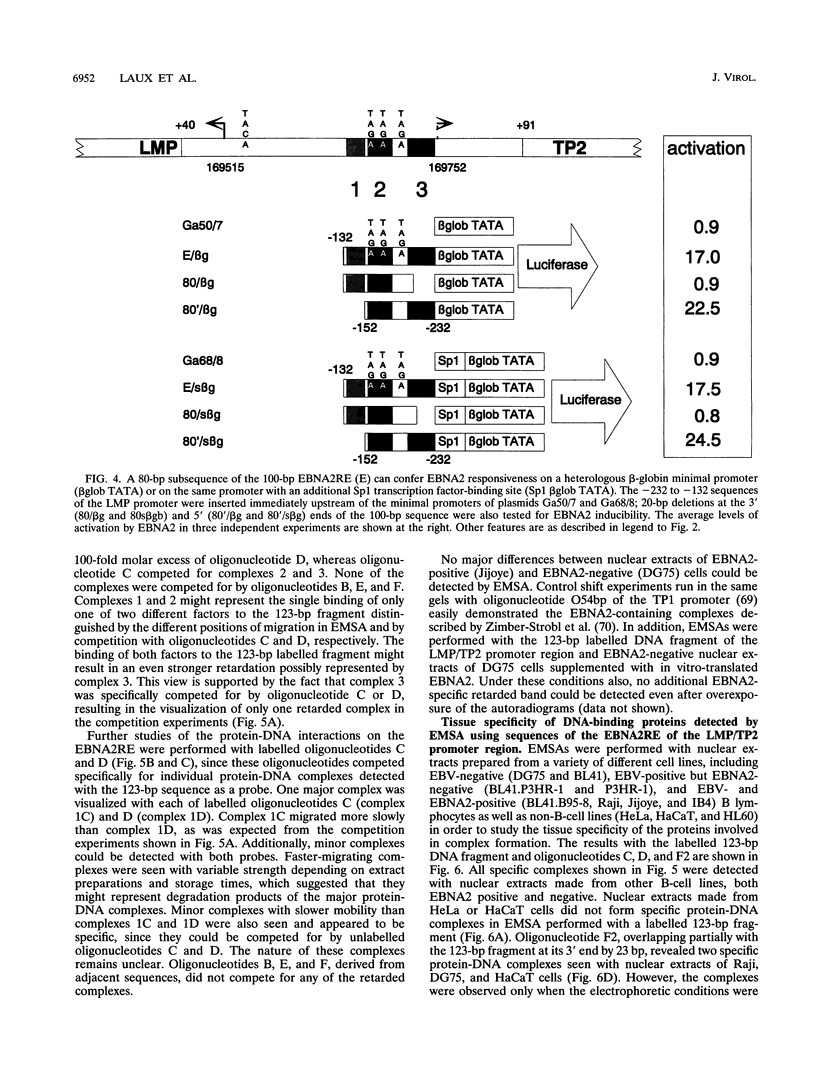
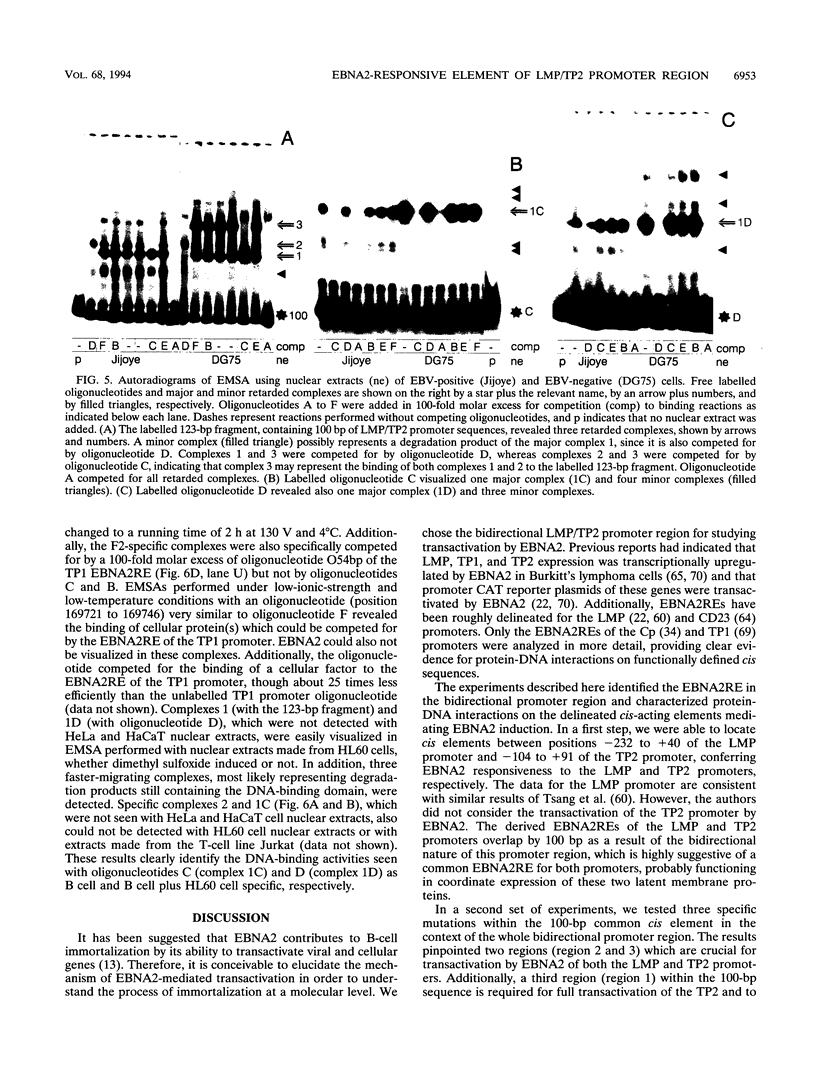
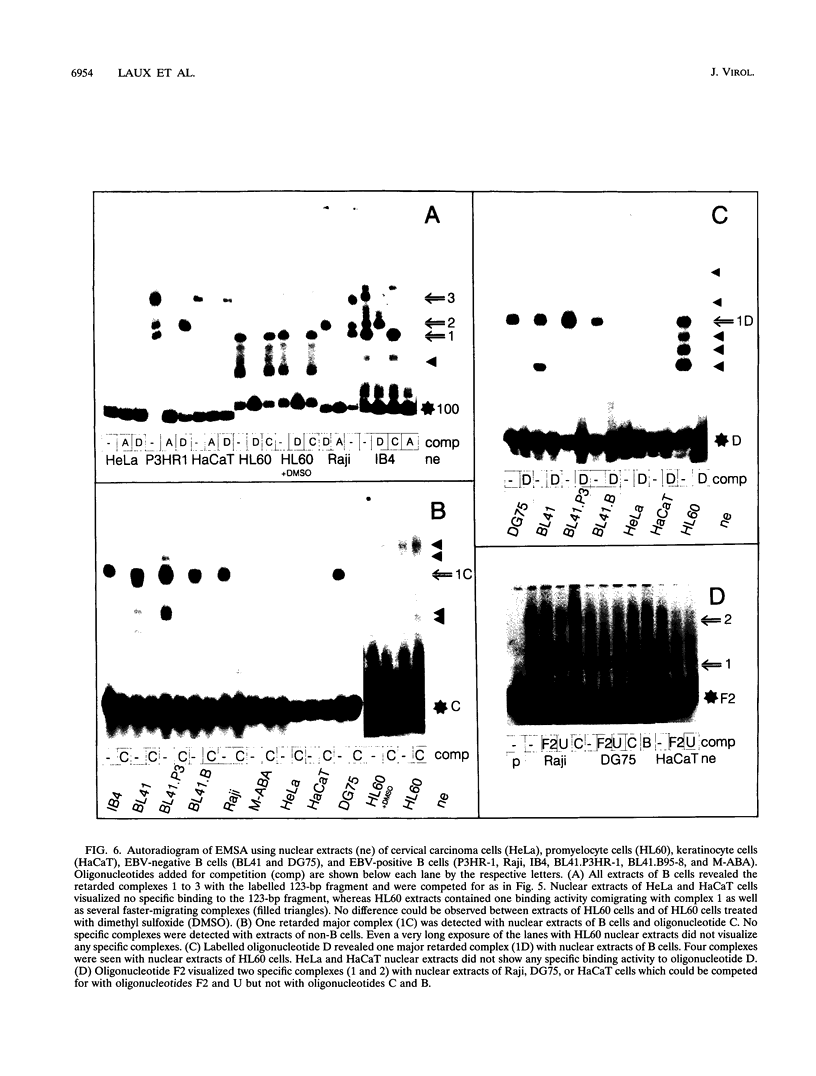
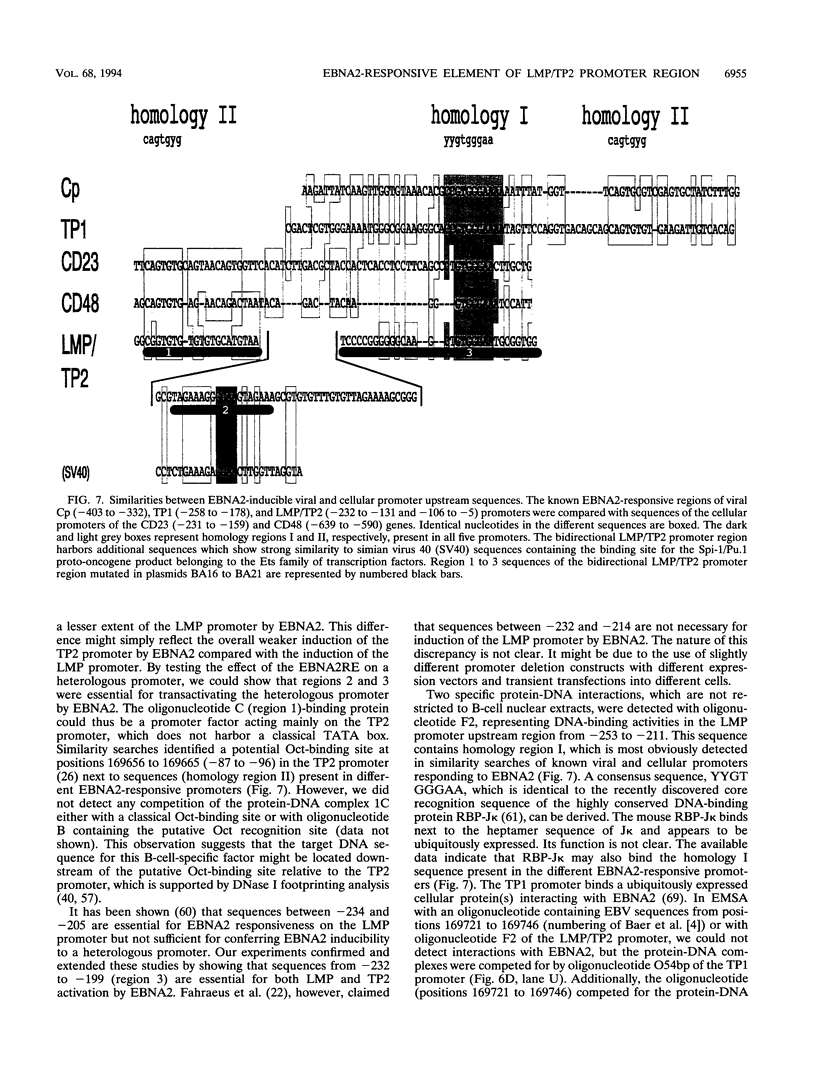
Epstein-Barr virus (EBV) transforms resting B cells in vitro very efficiently. The nuclear viral protein EBV nuclear antigen 2 (EBNA2) is absolutely required for this process and also acts as a transcriptional activator of cellular and viral genes. As shown previously, EBNA2 transactivates the promoters of the viral latent membrane proteins. It interacts indirectly with an EBNA2-responsive cis element of the terminal protein 1 (TP1) promoter. To identify the sequences mediating EBNA2 transactivation of the bidirectional promoter region driving expression of the latent membrane proteins LMP and TP2 in opposite directions, we assayed the effects of EBNA2 on the activities of promoter deletion and site-directed mutants of TP2 and LMP promoter luciferase reporter gene constructs by cotransfections into EBNA2-negative Burkitt's lymphoma cells. We were able to delineate an 80-bp EBNA2-responsive region (EBNA2RE) between -232 and -152 relative to the LMP RNA start site which could also mediate EBNA2-dependent activation on a heterologous promoter. Sequences of 20 and 32 bp located at the 5' and 3' ends, respectively, of the EBNA2RE were both essential for EBNA2 responsiveness. Full transactivation of the LMP and TP2 promoters seemed to require 20 bp of 5' adjacent sequences in addition to the 80-bp element. Electrophoretic mobility shift assays revealed specific protein-DNA complexes formed at the EBNA2RE. Oligonucleotides from -181 to -152 and -166 to -132 relative to the LMP RNA start site visualized one B-cell and one B-cell-plus-HL60-specific retarded protein-DNA complex, respectively. Additionally, an oligonucleotide from -253 to -210 revealed two specific protein-DNA complexes with nuclear extracts from different B and non-B cells, suggesting also the binding of ubiquitously expressed proteins on the EBNA2RE. Thus, these experiments defined a 80-bp cis element sufficient for conferring EBNA2 inducibility and demonstrated specific interactions of cellular proteins at DNA sequences within the EBNA2RE, which are critical for transactivation by EBNA2.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbot S. D., Rowe M., Cadwallader K., Ricksten A., Gordon J., Wang F., Rymo L., Rickinson A. B. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J Virol. 1990 May;64(5):2126–2134. doi: 10.1128/jvi.64.5.2126-2134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri C., Birkenbach M., Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991 Apr;181(2):595–608. doi: 10.1016/0042-6822(91)90893-g. [DOI] [PubMed] [Google Scholar]
- Allday M. J., Crawford D. H., Griffin B. E. Epstein-Barr virus latent gene expression during the initiation of B cell immortalization. J Gen Virol. 1989 Jul;70(Pt 7):1755–1764. doi: 10.1099/0022-1317-70-7-1755. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat H., Goldblum N., Mitrani S., Goldblum T., Yoffey J. M., Cohen M. M., Bentwich Z., Ramot B., Klein E., Klein G. Establishment in continuous culture of a new type of lymphocyte from a "Burkitt like" malignant lymphoma (line D.G.-75). Int J Cancer. 1977 Jan;19(1):27–33. doi: 10.1002/ijc.2910190105. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks L., Yao Q. Y., Rickinson A. B., Young L. S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992 May;66(5):2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson P., McCoy R., Sadler R., Gilligan K., Tursz T., Raab-Traub N. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992 May;66(5):3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Kieff E. An Epstein-Barr virus nuclear protein 2 domain essential for transformation is a direct transcriptional activator. J Virol. 1991 Nov;65(11):5880–5885. doi: 10.1128/jvi.65.11.5880-5885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Kieff E. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J Virol. 1991 May;65(5):2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Mannick J., Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Cordier-Bussat M., Billaud M., Calender A., Lenoir G. M. Epstein-Barr virus (EBV) nuclear-antigen-2-induced up-regulation of CD21 and CD23 molecules is dependent on a permissive cellular context. Int J Cancer. 1993 Jan 2;53(1):153–160. doi: 10.1002/ijc.2910530128. [DOI] [PubMed] [Google Scholar]
- Cordier-Bussat M., Calender A., Vuillaume M., Bornkamm G. W., Lenoir G. M. Expression of the Epstein-Barr virus (EBV) latent membrane protein is tightly regulated, independently of EB nuclear antigen 2 and of EBV integration or copy number. Virus Res. 1993 Jan;27(1):55–69. doi: 10.1016/0168-1702(93)90112-z. [DOI] [PubMed] [Google Scholar]
- Cordier M., Calender A., Billaud M., Zimber U., Rousselet G., Pavlish O., Banchereau J., Tursz T., Bornkamm G., Lenoir G. M. Stable transfection of Epstein-Barr virus (EBV) nuclear antigen 2 in lymphoma cells containing the EBV P3HR1 genome induces expression of B-cell activation molecules CD21 and CD23. J Virol. 1990 Mar;64(3):1002–1013. doi: 10.1128/jvi.64.3.1002-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. H., Epstein M. A., Bornkamm G. W., Achong B. G., Finerty S., Thompson J. L. Biological and biochemical observations on isolates of EB virus from the malignant epithelial cells of two nasopharyngeal carcinomas. Int J Cancer. 1979 Sep 15;24(3):294–302. doi: 10.1002/ijc.2910240305. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus R., Fu H. L., Ernberg I., Finke J., Rowe M., Klein G., Falk K., Nilsson E., Yadav M., Busson P. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer. 1988 Sep 15;42(3):329–338. doi: 10.1002/ijc.2910420305. [DOI] [PubMed] [Google Scholar]
- Fåhraeus R., Jansson A., Ricksten A., Sjöblom A., Rymo L. Epstein-Barr virus-encoded nuclear antigen 2 activates the viral latent membrane protein promoter by modulating the activity of a negative regulatory element. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7390–7394. doi: 10.1073/pnas.87.19.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus R., Jansson A., Sjöblom A., Nilsson T., Klein G., Rymo L. Cell phenotype-dependent control of Epstein-Barr virus latent membrane protein 1 gene regulatory sequences. Virology. 1993 Jul;195(1):71–80. doi: 10.1006/viro.1993.1347. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Kieff E. cis-acting regulatory elements near the Epstein-Barr virus latent-infection membrane protein transcriptional start site. J Virol. 1990 Apr;64(4):1855–1858. doi: 10.1128/jvi.64.4.1855-1858.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grässer F. A., Göttel S., Haiss P., Boldyreff B., Issinger O. G., Mueller-Lantzsch N. Phosphorylation of the Epstein-Barr virus nuclear antigen 2. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1694–1701. doi: 10.1016/s0006-291x(05)81604-3. [DOI] [PubMed] [Google Scholar]
- Grässer F. A., Haiss P., Göttel S., Mueller-Lantzsch N. Biochemical characterization of Epstein-Barr virus nuclear antigen 2A. J Virol. 1991 Jul;65(7):3779–3788. doi: 10.1128/jvi.65.7.3779-3788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath G. C., Schubach W. H. Identification of the Epstein-Barr virus nuclear antigen 2 transactivation domain. Biochem Biophys Res Commun. 1993 Feb 26;191(1):196–200. doi: 10.1006/bbrc.1993.1202. [DOI] [PubMed] [Google Scholar]
- Hu L. F., Minarovits J., Cao S. L., Contreras-Salazar B., Rymo L., Falk K., Klein G., Ernberg I. Variable expression of latent membrane protein in nasopharyngeal carcinoma can be related to methylation status of the Epstein-Barr virus BNLF-1 5'-flanking region. J Virol. 1991 Mar;65(3):1558–1567. doi: 10.1128/jvi.65.3.1558-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Farrell P. J., Barrell B. G. Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95-8. J Virol. 1985 Feb;53(2):528–535. doi: 10.1128/jvi.53.2.528-535.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X. W., Speck S. H. Identification of critical cis elements involved in mediating Epstein-Barr virus nuclear antigen 2-dependent activity of an enhancer located upstream of the viral BamHI C promoter. J Virol. 1992 May;66(5):2846–2852. doi: 10.1128/jvi.66.5.2846-2852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye K. M., Izumi K. M., Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Thomas-Powell A. L., Raab-Traub N., Hawke M., Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980 Nov;36(2):506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Knutson J. C. The level of c-fgr RNA is increased by EBNA-2, an Epstein-Barr virus gene required for B-cell immortalization. J Virol. 1990 Jun;64(6):2530–2536. doi: 10.1128/jvi.64.6.2530-2536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
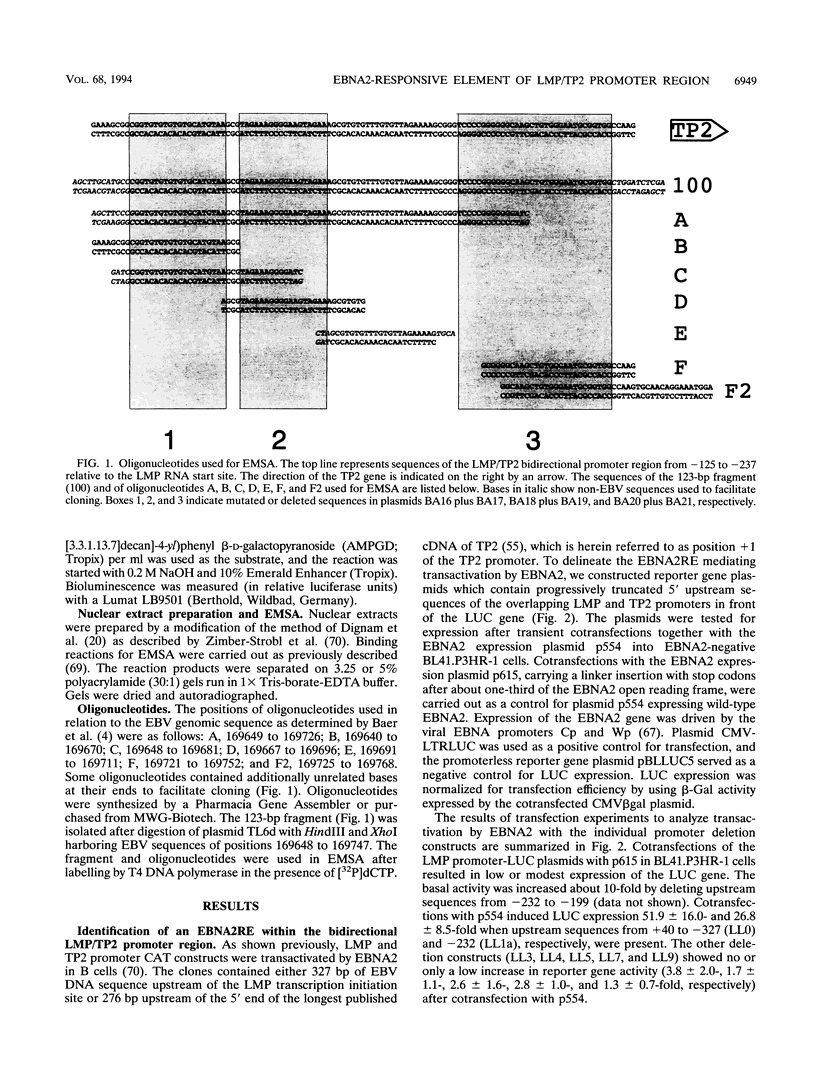
- Laux G., Economou A., Farrell P. J. The terminal protein gene 2 of Epstein-Barr virus is transcribed from a bidirectional latent promoter region. J Gen Virol. 1989 Nov;70(Pt 11):3079–3084. doi: 10.1099/0022-1317-70-11-3079. [DOI] [PubMed] [Google Scholar]
- Lenoir G. M., Vuillaume M., Bonnardel C. The use of lymphomatous and lymphoblastoid cell lines in the study of Burkitt's lymphoma. IARC Sci Publ. 1985;(60):309–318. [PubMed] [Google Scholar]
- Ling P. D., Rawlins D. R., Hayward S. D. The Epstein-Barr virus immortalizing protein EBNA-2 is targeted to DNA by a cellular enhancer-binding protein. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9237–9241. doi: 10.1073/pnas.90.20.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Green M. R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990 Jun 29;61(7):1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
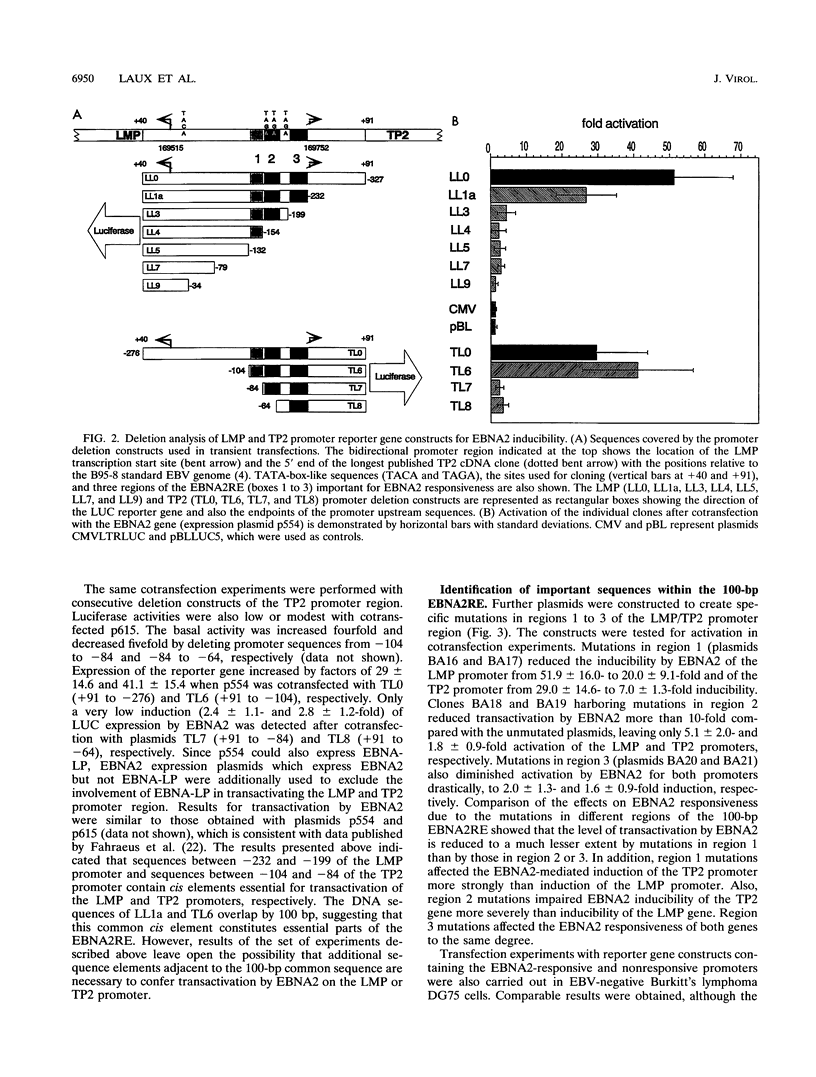
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minarovits J., Hu L. F., Imai S., Harabuchi Y., Kataura A., Minarovits-Kormuta S., Osato T., Klein G. Clonality, expression and methylation patterns of the Epstein-Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J Gen Virol. 1994 Jan;75(Pt 1):77–84. doi: 10.1099/0022-1317-75-1-77. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Sculley T. B., Pope J. H. Induction of Epstein-Barr virus nuclear antigens. J Virol. 1986 Jun;58(3):988–990. doi: 10.1128/jvi.58.3.988-990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudryj M., Devoto S. H., Hiebert S. W., Hunter T., Pines J., Nevins J. R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991 Jun 28;65(7):1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Sjöblom A., Masucci M. G., Rymo L. Viral and cellular factors influence the activity of the Epstein-Barr virus BCR2 and BWR1 promoters in cells of different phenotype. Virology. 1993 Apr;193(2):774–785. doi: 10.1006/viro.1993.1186. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C. M., Brimmell M., Buschle M., Allan G., Farrell P. J., Kolman J. L. Host cell and EBNA-2 regulation of Epstein-Barr virus latent-cycle promoter activity in B lymphocytes. J Virol. 1992 Jan;66(1):496–504. doi: 10.1128/jvi.66.1.496-504.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C., Howe J. G., Speck S. H., Miller G. Influence of Burkitt's lymphoma and primary B cells on latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J Virol. 1989 Apr;63(4):1531–1539. doi: 10.1128/jvi.63.4.1531-1539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Liebowitz D., Kieff E. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J Virol. 1989 Feb;63(2):933–937. doi: 10.1128/jvi.63.2.933-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung N. S., Kenney S., Gutsch D., Pagano J. S. EBNA-2 transactivates a lymphoid-specific enhancer in the BamHI C promoter of Epstein-Barr virus. J Virol. 1991 May;65(5):2164–2169. doi: 10.1128/jvi.65.5.2164-2169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B., Robertson E., Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J Virol. 1993 Apr;67(4):2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S. F., Wang F., Izumi K. M., Kieff E. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J Virol. 1991 Dec;65(12):6765–6771. doi: 10.1128/jvi.65.12.6765-6771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun T., Hamaguchi Y., Matsunami N., Furukawa T., Honjo T., Kawaichi M. Recognition sequence of a highly conserved DNA binding protein RBP-J kappa. Nucleic Acids Res. 1994 Mar 25;22(6):965–971. doi: 10.1093/nar/22.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Kikutani H., Tsang S. F., Kishimoto T., Kieff E. Epstein-Barr virus nuclear protein 2 transactivates a cis-acting CD23 DNA element. J Virol. 1991 Aug;65(8):4101–4106. doi: 10.1128/jvi.65.8.4101-4106.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Tsang S. F., Kurilla M. G., Cohen J. I., Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990 Jul;64(7):3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Gerster T., Müller M. M., Schaffner G., Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987 Sep 11;15(17):6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschlaeger M., Jin X. W., Yandava C. N., Furmanski L. A., Strominger J. L., Speck S. H. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3942–3946. doi: 10.1073/pnas.88.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- Zimber-Strobl U., Kremmer E., Grässer F., Marschall G., Laux G., Bornkamm G. W. The Epstein-Barr virus nuclear antigen 2 interacts with an EBNA2 responsive cis-element of the terminal protein 1 gene promoter. EMBO J. 1993 Jan;12(1):167–175. doi: 10.1002/j.1460-2075.1993.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimber-Strobl U., Suentzenich K. O., Laux G., Eick D., Cordier M., Calender A., Billaud M., Lenoir G. M., Bornkamm G. W. Epstein-Barr virus nuclear antigen 2 activates transcription of the terminal protein gene. J Virol. 1991 Jan;65(1):415–423. doi: 10.1128/jvi.65.1.415-423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]