Abstract
Patients infected with the human immunodeficiency virus type 1 (HIV-1) frequently develop central and peripheral nervous system complications, some of which may reflect the effect of the virus itself. In order to elucidate the pathogenic mechanisms of HIV in neurological disease in a small animal model, we generated transgenic mice expressing the entire HIV genome under control of the promoter for the human neurofilament NF-L gene. The transgene was predominantly expressed in anterior thalamic and spinal motor neurons. Animals developed a neurological syndrome characterized by hypoactivity and weakness and by axonal degeneration in peripheral nerves. These results provide evidence for a role of HIV in affecting both the central and peripheral nervous systems. This animal model may also facilitate the development of therapeutic agents against the human disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achim C. L., Heyes M. P., Wiley C. A. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J Clin Invest. 1993 Jun;91(6):2769–2775. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- BOND D. D., RANDT C. T., BIDDER T. G., ROWLAND V. Posterior septal, fornical, and anterior thalamic lesions in the cat; vegetative and behavioral changes with anatomical and physiological correlations. AMA Arch Neurol Psychiatry. 1957 Aug;78(2):143–162. doi: 10.1001/archneurpsyc.1957.02330380033003. [DOI] [PubMed] [Google Scholar]
- Bouchard L., Lamarre L., Tremblay P. J., Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989 Jun 16;57(6):931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Westbrook G. L., Fitzgerald S. P., Ennist D. L., Elkins K. L., Ruff M. R., Pert C. B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dreyer E. B., Kaiser P. K., Offermann J. T., Lipton S. A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990 Apr 20;248(4953):364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Fride E., Ben-Or S., Allweis C. Mitochondrial protein synthesis may be involved in long-term memory formation. Pharmacol Biochem Behav. 1989 Apr;32(4):873–878. doi: 10.1016/0091-3057(89)90051-8. [DOI] [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi R., Lebargy F., Gaulard P., Mhiri C., Bernaudin J. F., Gray F. Necrotizing vasculitis and HIV replication in peripheral nerves. N Engl J Med. 1989 Sep 7;321(10):685–686. doi: 10.1056/NEJM198909073211013. [DOI] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Glowa J. R., Panlilio L. V., Brenneman D. E., Gozes I., Fridkin M., Hill J. M. Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res. 1992 Jan 20;570(1-2):49–53. doi: 10.1016/0006-8993(92)90562-n. [DOI] [PubMed] [Google Scholar]
- Gravel C., Kay D. G., Jolicoeur P. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E murine leukemia virus. J Virol. 1993 Nov;67(11):6648–6658. doi: 10.1128/jvi.67.11.6648-6658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E., Naranjo J. N. Thalamic role in spatial memory. Behav Brain Res. 1986 Feb;19(2):123–131. doi: 10.1016/0166-4328(86)90010-0. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Bhat S., Spitalnik S. L., Laughlin M., Stefano K., Silberberg D. H., Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991 Jul 19;253(5017):320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
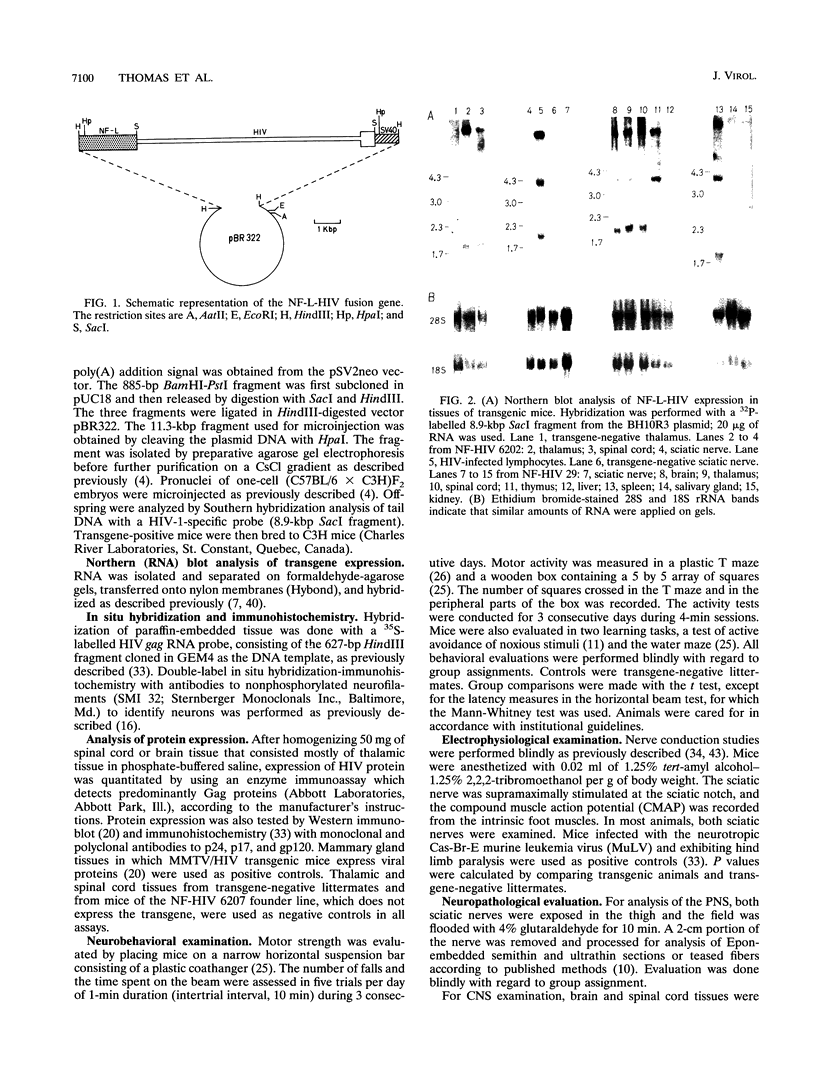
- Jolicoeur P., Laperrière A., Beaulieu N. Efficient production of human immunodeficiency virus proteins in transgenic mice. J Virol. 1992 Jun;66(6):3904–3908. doi: 10.1128/jvi.66.6.3904-3908.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J. P., Beaudet L., Tretjakoff I., Peterson A. Neurofilament gene expression in transgenic mice. J Physiol (Paris) 1990;84(1):50–52. [PubMed] [Google Scholar]
- Katsuki M., Sato M., Kimura M., Yokoyama M., Kobayashi K., Nomura T. Conversion of normal behavior to shiverer by myelin basic protein antisense cDNA in transgenic mice. Science. 1988 Jul 29;241(4865):593–595. doi: 10.1126/science.2456614. [DOI] [PubMed] [Google Scholar]
- Kay D. G., Gravel C., Pothier F., Laperrière A., Robitaille Y., Jolicoeur P. Neurological disease induced in transgenic mice expressing the env gene of the Cas-Br-E murine retrovirus. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4538–4542. doi: 10.1073/pnas.90.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Lalonde R., Joyal C. C., Botez M. I. Effects of folic acid and folinic acid on cognitive and motor behaviors in 20-month-old rats. Pharmacol Biochem Behav. 1993 Mar;44(3):703–707. doi: 10.1016/0091-3057(93)90188-y. [DOI] [PubMed] [Google Scholar]
- Lalonde R., Manseau M., Botez M. I. Spontaneous alternation and habituation in Purkinje cell degeneration mutant mice. Brain Res. 1987 May 12;411(1):187–189. doi: 10.1016/0006-8993(87)90699-8. [DOI] [PubMed] [Google Scholar]
- Lewis P., Hensel M., Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992 Aug;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER J. S., HUNTER J. Behaviour deficits following diencephalic lesions. Neurology. 1952 Mar-Apr;2(2):112–130. doi: 10.1212/wnl.2.5-6.112. [DOI] [PubMed] [Google Scholar]
- Mabrouk K., Van Rietschoten J., Vives E., Darbon H., Rochat H., Sabatier J. M. Lethal neurotoxicity in mice of the basic domains of HIV and SIV Rev proteins. Study of these regions by circular dichroism. FEBS Lett. 1991 Sep 2;289(1):13–17. doi: 10.1016/0014-5793(91)80898-d. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Cho E. S., Petito C. K., Price R. W. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986 Jun;19(6):525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Jordan B. D., Price R. W. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986 Jun;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Paquette Y., Kay D. G., Rassart E., Robitaille Y., Jolicoeur P. Substitution of the U3 long terminal repeat region of the neurotropic Cas-Br-E retrovirus affects its disease-inducing potential. J Virol. 1990 Aug;64(8):3742–3752. doi: 10.1128/jvi.64.8.3742-3752.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G. J., Cornblath D. R., Brown M. J. Transient conduction block following acute peripheral nerve ischemia. Muscle Nerve. 1985 Jun;8(5):409–412. doi: 10.1002/mus.880080510. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- SCHREINER L., RIOCH D. M., PECHTEL C., MASSERMAN J. H. Behavioral changes following thalamic injury in cat. J Neurophysiol. 1953 May;16(3):234–246. doi: 10.1152/jn.1953.16.3.234. [DOI] [PubMed] [Google Scholar]
- SCHULMAN S. IMPAIRED DELAYED RESPONSE FROM THALAMIC LESIONS. STUDIES IN MONKEYS. Arch Neurol. 1964 Nov;11:477–499. doi: 10.1001/archneur.1964.00460230027003. [DOI] [PubMed] [Google Scholar]
- Sabatier J. M., Vives E., Mabrouk K., Benjouad A., Rochat H., Duval A., Hue B., Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991 Feb;65(2):961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. M., Olney R. K. Peripheral neuropathies associated with human immunodeficiency virus infection. Neurol Clin. 1992 Aug;10(3):685–711. [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler M. H., Eskin T. A., Benn S., Angerer R. C., Angerer L. M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986 Nov 7;256(17):2360–2364. [PubMed] [Google Scholar]
- Thomas F. P., Thomas J. E., Sadiq S. A., van den Berg L. H., Roelofs R. I., Latov N., Hays A. P. Human monoclonal IgM anti-Gal(beta 1-3)GalNAc autoantibodies bind to the surface of bovine spinal motoneurons. J Neuropathol Exp Neurol. 1990 Mar;49(2):89–95. doi: 10.1097/00005072-199003000-00001. [DOI] [PubMed] [Google Scholar]
- Thomas F. P., Trojaborg W., Nagy C., Santoro M., Sadiq S. A., Latov N., Hays A. P. Experimental autoimmune neuropathy with anti-GM1 antibodies and immunoglobulin deposits at the nodes of Ranvier. Acta Neuropathol. 1991;82(5):378–383. doi: 10.1007/BF00296548. [DOI] [PubMed] [Google Scholar]
- Toggas S. M., Masliah E., Rockenstein E. M., Rall G. F., Abraham C. R., Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994 Jan 13;367(6459):188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Turnley A. M., Morahan G., Okano H., Bernard O., Mikoshiba K., Allison J., Bartlett P. F., Miller J. F. Dysmyelination in transgenic mice resulting from expression of class I histocompatibility molecules in oligodendrocytes. Nature. 1991 Oct 10;353(6344):566–569. doi: 10.1038/353566a0. [DOI] [PubMed] [Google Scholar]
- Werner T., Ferroni S., Saermark T., Brack-Werner R., Banati R. B., Mager R., Steinaa L., Kreutzberg G. W., Erfle V. HIV-1 Nef protein exhibits structural and functional similarity to scorpion peptides interacting with K+ channels. AIDS. 1991 Nov;5(11):1301–1308. doi: 10.1097/00002030-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gans J., Portegies P. Neurological complications of infection with human immunodeficiency virus type 1. A review of literature and 241 cases. Clin Neurol Neurosurg. 1989;91(3):199–219. doi: 10.1016/0303-8467(89)90114-5. [DOI] [PubMed] [Google Scholar]